* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Prescribing in Pregnancy_2011
Compounding wikipedia , lookup
Plateau principle wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Orphan drug wikipedia , lookup
Drug design wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Psychopharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
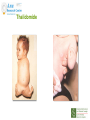
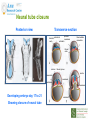
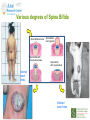
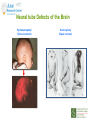
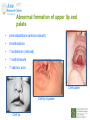
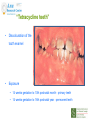
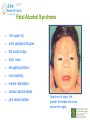
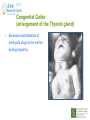
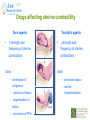
Prescribing in Pregnancy Prof Louise Kenny PhD MRCOG Professor of Obstetrics and Consultant Obstetrician and Gynaecologist Cork University Maternity Hospital UCC Aims • Awareness of physiology of pregnancy • How pregnancy alters pharmacokinetics • Placental transfer • Teratology • Drugs utilised in pregnancy Terms and definitions • Pharmacokinetics – Study of the time course of drug absorption, distribution, metabolism and excretion • Pharmacodynamics – Study of the biochemical and physiological effects of drugs and their mechanisms of action Terms and definitions • Plasma half-life – The time taken for drug concentration to fall by 50% • Steady state – Occurs when the amount of the drug administered during the dosage interval equals the amount of drug eliminated during the dosage interval. This is dependent on the half-life. The actual steady state concentration is directly proportional to the size of the dose and indirectly proportional to the volume of distribution and the eliminator constant Terms and definitions • Clearance – The volume of plasma cleared of drug in unit time (ml/min) • First pass effect – Metabolic breakdown of drugs before entering the systemic circulation on iniial passage through the liver (e.g. lidocaine, pethidine and oestrogen), the intestinal mucosa (e.g. L-dopa, chlorpromazine and ethanol) or lungs (e.g. isoprenaline). Terms and definitions • Bioavailability – Extent to which a drug is absorbed systemically. It is dependent upon tablet formulation, gut motility, disease states and first pass effect. • Volume of distribution – The theoretical fluid volume which would contain the total body content of a drug at a concentration equal to the plasma concentration. Drugs that are highly lipophilic and extensively tissue bound have a large volume of distribution (e.g. nortriptyline) Terms and definitions • Drug elimination – First order (linear) elimination. • Most common type for drug absorption and elimination. • The rate of reaction is directly proportional to the amount of drug available (i.e. constant fraction of drug absorbed or eliminated in unit time) – Zero order elimination • Proceeds at a constant rate independent of the amount of drug available. Drug concentration Terms and definitions first order kinetics zero order kinetics Dose Pharmacokinetics and Pharmacodynamics in Pregnancy Physiological changes of Pregnancy • in plasma volume • in cardiac output • in renal blood flow and GFR • Induction of liver enzyme pathways • in plasma protein content • Delayed gastric emptying Resulting changes in pharmacokinetics • volume of distribution • plasma concentration • excretion renal excretion • hepatic metabolism Placental Transfer • Golden rule is that every drug crosses the placenta and under normal circumstances most drugs equilibrate between maternal and fetal compartments. The only exception is heparin, which because of its large molecular weight and polarity, does not cross Teratology (the study of monsters) Teratogens • An agent that causes physical and/or developmental abnormalities by preventing the developing embryo or fetus reaching its full genetic potential Potential of drug teratogenicity • dose • exposure time • bioavailability • degradation products • drug interactions Drugs in Pregnancy • Most pregnancies are unexpected • Even ‘planned’ pregnancies are not detected until 8-10 weeks gestation • Many women take vitamin supplements and over the counter medications The Lesson of Thalidomide Thalidomide History • Synthesised by Ciba in 1953 • Taken over by Chemie Gruenthal in 1954 • Trials indicated it had mild sedative properties • Marketed in 1957 for nausea and morning sickness • “drug of choice to help pregnant women” • Known as Contergan but also incorporated into many over the counter preparations • Licensed in Europe and Australia and Japan History II • First affected child born in \West Germany in 1956 • Dr McBride in Sydney Australia published a letter in Lancet in December 1961 • Rare limb and ear defects noticed in unprecedented numbers • 60% of mothers gave a history of taking Contergan • Drug was withdrawn between 1962-1963 • 10,000 affected children were born world-wide • 40% of victims died before their first birthday Evidence of teratogenicity of a drug • double blind randomised control trials in humans • isolated case reports • epidemiological studies • laboratory experiments Laboratory experimental work • testing on cell lines • testing on tissue culture models • in vitro embryo culture models – e.g. whole rat embryo culture • pregnant mammalian studies Limitations of laboratory experimental work • what dose to study? • what exposure window? • which experimental model to use? • does evidence of toxicity provide evidence of teratogenicity? • different species sensitivity and bioavailability • Classic example - thalidomide! Early development 1 2 Main embryonic period (weeks) 3 4 5 6 7 Neural tube defects 8 9 Cleft lip 16 CNS LIMBS UPPER LIP Microphthalmia, cataracts,glaucoma Enamel hypoplasia Cleft palate Masculinsation Common site(s) of action 38 HEART Low-set malformed ears and deafness Embryo Death 32 Mental retardation TA, ASD, and VSD Amelia/Meromelia Fetal period (weeks) Major congenital anomalies Highly sensitive period EARS EYES TEETH PALATE GENITALIA Functional & minor anomalies Less sensitive period Abnormal limb development secondary to thalidomide ingested by pregnant mother • Thalidomide is a tranquiliser, • sedative & immunosuppressant • Critical exposure window • 24 to 36 days post fertilisation • Defects : – amelia - no limbs – micromelia - short limbs – cardiac defects – haemangiomas – defects of urinary tract – defects of digestive tract FDA Classification of Drugs Risk to Fetus • Cat A – Controlled studies in women fail to demonstrate a risk to the fetus in the 1st trimester (and there is no evidence of risk in later pregnancy) and the possibility of fetal harm is remote • Cat B – Either animal studies have not demonstrated a fetal risk but there are no controlled studies in women, or animal studies have shown an adverse effect but this has not been confirmed in controlled studies in women • Cat C – Either studies in animal have shown an adverse fetal effect and there are no contolled studies in women or studies in women and animals are not available FDA Classification of Drugs Risk to Fetus • Cat D – There is positive evidence of an adverse risk in the human fetus but the benefits from use in pregnant women may outweigh the risk • Cat X – Studies in animals or humans have demonstrated significant fetal abnormalities and the risk of the use of the drug in pregnant women clearly outweighs any potential benefit Anticonvulsants • Phenytoin – craniofacial abnormalities – hypoplasia of distal phalanges – growth deficiency – mental deficiency • Valproic acid – associated with neural tube defects • Carbamazepine (Tegretol) – similar to phenytoin but decreased risk and therefore drug of choice Neural tube closure Posterior view Transverse section Amniotic cavity paraxial mesoderm ectoderm notochord Intermediate mesoderm Dorsal aorta amnion Neural groove somite mesoderm mesoderm endoderm mesoderm Developing embryo day 17 to 21 Showing closure of neural tube endoderm Various degrees of Spina Bifida Spina bifida occulta Spina bifida with meningomyelocele Spina bifida meningocele Spina bifida with myeloschisis Normal lower limbs Affected lower limbs Neural tube Defects of the Brain Hydranencephaly Anencephaly (Closed cranium) (Open cranium) Formation of the upper lip and palate Later nasal prominence Maxillary prominence Medial nasal prominence Inferior view of palate showing fusion of palatal plates of maxillary prominences Fusion of facial bones Scanning electron micrograph of facial bones before fusion Abnormal formation of upper lip and palate • phenolbarbitone (anticonvulsant) • trimethadione • ? isotretinoin (retinoid) • ? methotrexate • ? valproic acid Cleft palate Cleft lip & palate Cleft lip “Tetracycline teeth” • Discolouration of the tooth enamel • Exposure • 14 weeks gestation to 10th postnatal month - primary teeth • 14 weeks gestation to 16th postnatal year - permanent teeth Genital abnormality (8 -10 weeks) FEMALE MALE • Genital tubercle (GT) - Clitoris Penis • Genital swelling (GS) - Labia majora Scrotum • Urethral fold (UF) - Labia minora Dorsum of penis • Anal fold (AF) - Anus Anus GT GS UF Masculinisation of female genitalia (enlarged clitoris and labia majora) AF Fetal Alcohol Syndrome • thin upper lip • short palpebral fissures • flat nasal bridge • short nose • elongated philtrum • microcephaly • mental retardation • cardiac abnormalities • joint abnormalities Spectrum of signs, the greater the intake the more severe the signs Congenital Goiter (enlargement of the Thyroid gland) • Excessive administration of antithyroid drugs to the mother during pregnancy Drugs affecting uterine contractility Toco agents • ↑ strength and Tocolytic agents • ↓ strength and frequency of uterine frequency of uterine contractions contractions Uses Uses – termination of pregnancy – induction of labour – augmentation of labour – prevention of PPH – premature labour – uterine hyperstimulation Drugs affecting uterine contractility • Toco agents • Tocolytic agents • oxytocin • ergometrine • prostaglandin – analgesics • mifepristone – calcium antagonists • misoprostol – indamethacin • carboprost – Atosiban (oxytocin receptor – Ritodrine (β2adrenoceptor agonist) antagonist) Coagulation and pregnancy • Pregnancy produces a thrombophilic state in the mother • helps prevent post partum haemorrhage • thrombophilia, decreased venous return due to the gravid uterus and immobility during labour Risk of DVT and Pulmonary embolus • Warfarin is Teratogenic – chondroplasia punctata – optic atrophy – mental retardation Heparin and Low molecular weight heparins are safe Cytotoxic Drugs Vinca alkaloids Vincristine Joint pain Paralytic ileus Alkylating agents Peripheral neuropathy Cyclophosphamide Vinblastine Haemmorhagic cystitis Infertility Antimetabolites M Methotrexate Skin pigmentation G0 Busulphan G2 Heptatotxicity Aplastic anaemia Pneumonitis Skin pigmentation Encephalopathy cell cycle Azothiaprine Hepatitis Lymphoma Pulmonary fibrosis G1 Daunorubicin Myocardial toxicity (also Adriamycin and S Doxorubicin) 6-Mercaptopurine Adriamycin 5-fluoruracil Chlorambucil Actinomycin-D Steroids G0= latent phase; G1= resting pase; G2 premitotic phase; S= synthesis of DNA; M= mitosis and division Drugs and Lactation • Drugs excreted in breast milk – Antibiotics, e.g. aminoglycosides, sulphonamides, tetracycline, metronidazole, chloramphenicol – Anti-TB drugs, e.g. isoniazid – CNS drugs, e.g. narcotic analgesics, benzidiazepines, chlorpromazine – Anti-thyroid drugs, e.g. carbimazole, radioactive iodine – Anti-convulsant drugs, e.g. phenytoin, phenobarbitone – Anticoagulation, e.g. phenindiones (warfarin and heparin) – Cytotoxic drugs and high dose corticosteroids Drugs and the endocrine system • Galactorrhoea – Methyldopa, L-dopa, phenothiazines, metoclopramide, cimetidine, benzodiazepines, oestrogens, tricyclic antidepressants • Gynaecomastia – Spironolactone, cimetidine, methyldopa, phenothiazines, tricyclic antidepressants, cytotoxic drugs, digoxin, oestrogens • Hypothyroidism – Iodides, lithium, amiodarone • Vaginal carcinoma – Diethystilboestrol