* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Incidence and Diversity of Plant Viruses
Plant nutrition wikipedia , lookup
History of botany wikipedia , lookup
Plant stress measurement wikipedia , lookup
Plant defense against herbivory wikipedia , lookup
Plant evolutionary developmental biology wikipedia , lookup
Plant use of endophytic fungi in defense wikipedia , lookup
Plant physiology wikipedia , lookup
Plant secondary metabolism wikipedia , lookup
Plant breeding wikipedia , lookup
Plant morphology wikipedia , lookup
Glossary of plant morphology wikipedia , lookup
Sustainable landscaping wikipedia , lookup
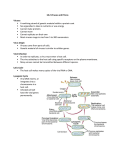
The Incidence and Diversity of Plant Viruses in the Tallgrass Prairie Preserve -Vaskar Thapa, Ulrich Melcher, Daniel McGlinn, Marilyn Roossink, Drew Porter, Rita Marvelli, Tracy Feldman, and Michael Palmer Outline 1) What is PVBE? 2) Methods • Field methods • Laboratory methods 3) Results • Incidence of plant viruses • Diversity of plant viruses 4) Summary Background: What is PVBE? • Plant Virus Biodiversity and Ecology is one of two scientific theme areas, awarded for research by the National Science Foundation (NSF)-Oklahoma Experimental Program to stimulate Competitive Research (EPSCoR). • Funding provided 2005 to 2008. Objective for PVBE To discover diversity and ecological functions of plant viruses in natural systems. Underlying hypothesis The distribution and evolution of viruses are determined by complex environmental interactions among many factors including distributions of hosts, vectors, other viruses and climate. Working team University of Tulsa joining soon Specializing in Ecology Virology Molecular biology Genomics Structural biology and Bioinformatics. Research site The Nature Conservancy’s Tallgrass Prairie Preserve in Osage County, Oklahoma • Representation of intact native Tallgrass Prairie landscape • 15,000 hectares • more than 700 plant species • 12 vegetation types Palmer, M. W., P. Earls, and J. R. Arévalo. 2000. The vegetation of the Tallgrass Prairie Preserve (unpublished report). Focus of this presentation Preliminary results from plants collected since May 2005 Analysis of double-stranded (ds) RNA from the plant collected Methods Field methods Plants for voucher herbarium Sample collection for dsRNA isolation Plant samples for intensive study Laboratory methods ds RNA isolation Field methods Plant sample for voucher herbarium • Sample from each species • Collection irrespective of symptoms • Collection from sites with abundance of target species • Record of GPS location • Habitat and individual plant photos • Two repositories for herbarium –OSU and TGPP Field methods .. Sample collection for ds RNA isolation • 10 grams of young leaves • Transported to the laboratory in a container with ice packs • Stored in cold room at 4 ْ C before processing for dsRNA isolation Field methods .. Plant samples for intensive study • Six of the most frequent plants in tallgrass prairie vegetation • Represent major taxonomic groups • Multiple samples from 20 random plots Ambrosia psilostachya, Cuman ragweed Asclepias viridis, green antelopehorn Panicum virgatum, switchgrass Sorghastrum nutans, Indiangrass Ruellia humilis, fringeleaf wild petunia Vernonia baldwini, baldwin's ironweed Laboratory method for double-stranded RNA isolation Mix vigorously to form emulsion Young leaves (5 g) Centrifuge Grind in liquid Transfer into 50 ml tube nitrogen containing 10 ml extraction buffer and 10 ml Ph:Ch Transfer top phase into new tube Final aqueous phase Repeat Ph:Ch extraction Decant and resuspend in 0,1 mM EDTA / 0.3 M NaOAC Centrifuge to pellet dsRNA Wash in 6 time with application buffer Precipitate with NaOAc and EtOH overnight at -20ْ C Transfer eluate to a 15 ml tube 1 2 3 4 Lad 1 kb Transfer to a microcentrifuge tube and fill with cold ethanol to reprecipitate Add absolute proof ethanol (16,5% of aqueous volume) Add elution buffer Resuspend in 50 mkl 0.1 mM EDTA bp 12,2 2 1,6 Check the dsRNA by electrophoresis on a 1.5% agarose gel in 0.5X TBE II Pass through enocolumn containing CF11powder cellulose binding dsRNA (if ethanol concentration is 16,5%) Vernonia baldwinii (line 1) and Flavoparmelia sp. (line 4) have no dsRNA. Total NA (for bar coding and making hybridization target) Buffers Extraction buffer: Application buffer: 0.1 M NaCl 0.1 M NaCl 50 mV Tris, pH 8 50 mM Tris, pH8 1 mV EDNA, pH 8 0.5 mV EDTA, pH8 1% SDS 16.5% Ethanol Elution buffer: 0.1 M NaCl 50 mV EDTA, pH 8 1 506,5 396 344 298 Ambrosia psilostachya (line 2) and Parmelia sp1. (line 3) show bands for dsRNA Protocol for ds RNA isolation adopted from M. Roossinck, 2005 Results • 635 specimen from 485 species, 307 genera and 91 families collected. • 592 specimens analyzed for 48% 52% ds RNA • gels of 592 these specimens • 308 of the are putatively Positive for dsRNA positive for dsRNA (i.e. probable viruses) Negative for dsRNA sc le pi ad ac ea e Cy pe ra ce ae A ca nt he ce ae Fa ba ce ae pi ac ea e 300 250 Po ac ea e A ste ra ce 84 ae ot he rf am ili es A A Number of specimen Distribution within the top plant families 2 = 37.39, p = 0.00 Negative Positive 200 150 100 50 0 Double-stranded RNA in native and exotic species 2 = 0.06, p = 0.8 120% Percentage of specimen 100% 80% Negative 60% Positive 40% 20% 0% Native Exotic Nativity Distribution of dsRNA in different life forms 2 = 1.23, p = 0.87 120% Percentage of specimen 100% 80% Negative 60% Positive 40% 20% 0% Perennial Annual Tree Life forms Shurb Vine Distribution of dsRNA in different taxonomic groups 2 = 13.81, p = 0.00 120% Percentage of specimen 100% 80% Negative 60% Positive 40% 20% 0% Dicot Monocot Fern Distribution of dsRNA in six selected species 2 = 9.76, p = 0.08 Ruellia humilis Vernonia baldwinii Asclepias viridis Positive Negative Sorghastrum nutans Panicum virgatum Ambrosia psilostachya 0% 20% 40% 60% 80% 100% 120% Viral Diversity • Too early to comment on plant virus diversity in TGPP • Gel analysis shows wide variation in banding patterns • Different banding patterns within and across species. Caveats • The results are preliminary, based on a limited sample • dsRNA is not unique for plant virus, it may be from fungal or arthropod viruses • Viruses of low titer may have been missed • DNA viruses are not assessed. • The reading of the gels has some subjectivity; this will be resolved in the sequencing phase of PVBE Conclusions • 50% of plant samples contain dsRNA, indicating viruses are widespread in nature. • Viruses are frequent in all growth forms, life histories, and taxonomic groups. Acknowledgments Following persons who help us in plant collection Pete Earls Fumiko Shirakura M.Hara Will Lowry Shyam Thomas Ray Moranz Josh Lofton Mari Carmen Cobo Laxman Karki Katie Lewis Rest of all team members of PVBE