* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture 2: Applications of Tissue Culture to Plant
Cultivated plant taxonomy wikipedia , lookup
Ornamental bulbous plant wikipedia , lookup
Venus flytrap wikipedia , lookup
History of botany wikipedia , lookup
Plant use of endophytic fungi in defense wikipedia , lookup
Plant secondary metabolism wikipedia , lookup
Plant physiology wikipedia , lookup
Sustainable landscaping wikipedia , lookup
Hybrid (biology) wikipedia , lookup
Flowering plant wikipedia , lookup
Plant morphology wikipedia , lookup
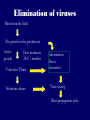
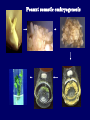
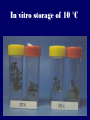
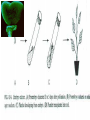
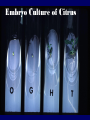
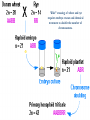
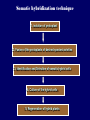
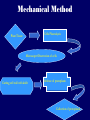
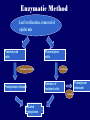
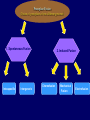
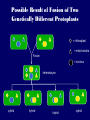
Plant Tissue Culture Application Development of superior cultivars Germplasm storage Somaclonal variation Embryo rescue Ovule and ovary cultures Anther and pollen cultures Callus and protoplast culture Protoplasmic fusion In vitro screening Multiplication Tissue Culture Applications Micropropagation Germplasm preservation Somaclonal variation Haploid & dihaploid production In vitro hybridization – protoplast fusion Micropropagation Micropropagation advantages From one to many propagules rapidly Multiplication in controlled laboratorium conditions Continuous propagation year round Potential for disease-free propagules Inexpensive per plant once established Precise crop production scheduling Reduce stock plant space Micropropagation disadvantages Specialized equipment/facilities required More technical expertise required Protocols not optimized for all species Plants produced may not fit industry standards Relatively expensive to set up Micropropagation applications Rapid increase of stock of new varieties Elimination of diseases Cloning of plant types not easily propagated by conventional methods (few offshoots/ sprouts/ seeds; date palms, ferns) Propagules have enhanced growth features (multibranched character) Methods of micropropagation Axillary branching • >95% of all micropropagation • Genetically stable • Simple and straightforward • Efficient but prone to Adventitious shoot genetic instability formation (organogenesis) Somatic embryogenesis • Little used. Potentially phenomenally efficient Axillary shoot proliferation Growth of axillary buds stimulated by cytokinin treatment; shoots arise mostly from pre-existing meristems Clonal in vitro propagation by repeated enhanced formation of axillary shoots from shoottips or lateral meristems cultured on media supplemented with plant growth regulators, usually cytokinins. Shoots produced are either rooted first in vitro or rooted and acclimatized ex vitro Steps of micropropagation (axillary branching and adventitious shoot formation) • Stage 0 – Selection & preparation of the mother plant Sterilization of the plant tissue • Stage I - Initiation of culture Explants placed into growth media • Stage II - Multiplication Explants transferred to shoot media; shoots can be constantly divided • Stage III - Rooting Explants transferred to root media • Stage IV - Transfer to soil Explants returned to soil; hardened off Clean Stock Program Used for Commercial Potato Procedures for cleaning virus infected clones and subsequent generation of nuclear seed potatoes for distribution Seed Potato Production A C B D Shoots (A) from virus-free merstems multiplied in vitro (B) are transferred into soil medium and grown in a screened greenhouse (C, D) to ward off insect vectors Ways to eliminate viruses Heat treatment. Plants grow faster than viruses at high temperatures. Meristemming. Viruses are transported from cell to cell through plasmodesmata and through the vascular tissue. Apical meristem often free of viruses. Trade off between infection and survival. Not all cells in the plant are infected. Adventitious shoots formed from single cells can give virusfree shoots. Elimination of viruses Plant from the field Pre-growth in the greenhouse Active growth Heat treatment 35oC / months ‘Virus-free’ Plants Meristem culture Adventitious Shoot formation Virus testing Micropropagation cycle Somatic Embryogenesis Explant → Callus Embryogenic → Maturation → Germination 1.Callus induction 2. Embryogenic callus development 3.Maturation 4.Germination Induction • Auxins required for induction – Proembryogenic masses form – 2,4-D most used – NAA, dicamba also used Development Auxin must be removed for embryo development Continued use of auxin inhibits embryogenesis Stages are similar to those of zygotic embryogenesis – – – – – Globular Heart Torpedo Cotyledonary Germination (conversion) Maturation • Require complete maturation with apical meristem, radicle, and cotyledons • Often obtain repetitive embryony • Storage protein production necessary • Often require ABA for complete maturation • ABA often required for normal embryo morphology – Fasciation – Precocious germination Germination • May only obtain 3-5% germination • Sucrose (10%), mannitol (4%) may be required • Drying (desiccation) – ABA levels decrease – Woody plants – Final moisture content 10-40% • Chilling – Decreases ABA levels – Woody plants Peanut somatic embryogenesis Plant germplasm preservation In situ : Conservation in ‘normal’ habitat –rain forests, gardens, farms Ex Situ : –Field collection, botanical gardens –Seed collections –In vitro collection: Extension of micropropagation techniques •Normal growth (short term storage) •Slow growth (medium term storage) •Cryopreservation (long term storage) DNA Banks In vitro Collection Potential advantages of in vitro methods: little space needs plants are free of pests, pathogens and viruses (and will remain so) no transfer labor (under storage conditions) stored cultures can be used as nuclear stock for vegetative preservation international shipping restrictions are lessened 1. no soil 2. pest-free plants In vitro Collection • Basic goals of an in vitro storage system – to maintain genetic stability – to keep in indefinite storage without loss of viability – must be economical • Two/three types of systems: – Normal growth – Slow growth – Cryopreservation Normal Growth 1. It can be done either on semi solid media or in liquid media 2. It is similar to multiplication stage in micropropagation 3. It must be frequently sub-cultured 4. When axillary buds are used as explants, it is considered as genetically stabile Slow growth It can store at least 1 semester and maximum 6 years without sub-culturing Ways to achieve slow growth: Addition of inhibitors or retardants Increasing osmotic potential of the media Manipulating storage temperature and light (cold storage (1-9° C)) Reducing light intensity Mineral oil overlay (callus) Reduced oxygen tension Plant Growth Retardants any chemicals that slow cell division and elongation in shoot tissues Cause plants to be shorter and more compact Interrupts cell division, stem elongation, and seed head formation Roots continue to grow May reduce the natural Gibberellic acid May produce more ethylene Cold storage storage at non-freezing temps, from 1-9° C dependent on species. storage of shoot cultures (stage I or II) • works well for strawberries, potatoes, grapes, prob. many more spp. • transferred (to fresh medium) every 6 month or on a yearly basis Advantages: simple, high rates of survival, useful for micro-propagation (especially in periods of low demand) Disadvantages • It may not be suitable for tropical, subtropical species because of susceptibility of these to chill injury • It is an alternative with coffee – shoot cultures transferred to a medium with reduced nutrients and lacking sucrose • It requires refrigeration, which is more expensive than storage in cryopreservation In vitro storage of 10 C Cryopreservation Storage of living tissues at ultra-low temperatures (-196°C) Conservation of plant germplasm • Vegetatively propagated species (root and tubers, ornamental, fruit trees) • Recalcitrant seed species (Howea, coconut, coffee) Conservation of tissue with specific characteristics • Medicinal and alcohol producing cell lines • Genetically transformed tissues • Transformation/Mutagenesis competent tissues (ECSs) Eradication of viruses (Banana, Plum) Conservation of plant pathogens (fungi, nematodes) Cryopreservation Steps Selection Excision of plant tissues or organs Culture of source material Select healthy cultures Apply cryo-protectants Pre-growth treatments Cooling/freezing Storage Warming & thawing Recovery growth Viability testing Post-thawing Cryopreservation Requirements • Preculturing – Usually a rapid growth rate to create cells with small vacuoles and low water content • Cryoprotection – Cryoprotectant (Glycerol, DMSO/dimetil sulfoksida, PEG) to protect against ice damage and alter the form of ice crystals • Freezing – The most critical phase; one of two methods: • Slow freezing allows for cytoplasmic dehydration • Quick freezing results in fast intercellular freezing with little dehydration Cryopreservation Requirements • Storage – Usually in liquid nitrogen (-196oC) to avoid changes in ice crystals that occur above -100oC • Thawing – Usually rapid thawing to avoid damage from ice crystal growth • Recovery – Thawed cells must be washed of cryo-protectants and nursed back to normal growth – Avoid callus production to maintain genetic stability Somaclonal Variation Variation found in somatic cells dividing mitotically in culture A general phenomenon of all plant regeneration systems that involve a callus phase Variation in trait(s) generated by use of a tissue-culture cycle Genetic variations in plants that have been produced by plant tissue culture and can be detected as genetic or phenotypic traits Two general types of Somaclonal Variation: – Heritable, genetic changes (alter the DNA) – Stable, but non-heritable changes (alter gene expression, epigenetic) Genetic (Heritable Variations) • Pre-existing variations in the somatic cells of explant • Caused by mutations and other DNA changes • Occur at high frequency Epigenetic (Non-heritable Variations) • Variations generated during tissue culture • Caused by temporary phenotypic changes • Occur at low frequency Causes of Somaclonal Variations Biochemical Cause Physiological Cause Genetic Cause Genetic Cause 1. 2. 3. 4. 5. 6. Change in chromosome number Change in chromosome structure Gene Mutation Extrachomosomal gene mutation Transposable element activation DNA sequence DNA sequence Change in DNA Detection of altered fragment size by using Restriction enzyme Change in Protein Loss or gain in protein band Alteration in level of specific protein Methylation of DNA Methylation inactivates transcription process Advantages of Somaclonal Variations • Help in crop improvement • Creation of additional genetic varitaions • Increased and improved production of secondary metabolites • Selection of plants resistant to various toxins, herbicides, high salt concentration and mineral toxicity • Suitable for breeding of perrenial species Disadvantages of Somaclonal Variations • A serious disadvantage occurs in operations which require clonal uniformity, as in the horticulture and forestry industries where tissue culture is employed for rapid propagation of elite genotypes • Sometime leads to undesirable results • Selected variants are random and genetically unstable • Require extensive and extended field trials • Not suitable for complex agronomic traits like yield and quality • May develop variants with pleiotropic effects which are not true. Somaclonal Breeding Procedures • Use plant cultures as starting material – Idea is to target single cells in multi-cellular culture – Usually suspension culture, but callus culture can work (want as much contact with selective agent as possible) – Optional: apply physical or chemical mutagen • Apply selection pressure to culture – Target (very high kill rate) – Generate screening dosage (lethal dosage is dependent upon the expected number survive cells • Regenerate whole plants from surviving cells - Direct organogenesis or embryogenesis Requirements for Somaclonal Breeding • Effective screening procedure – Most mutations are deleterious • With fruit fly, the ratio is ~800:1 deleterious to beneficial – Most mutations are recessive • Must screen M2 or later generations • Consider using heterozygous plants? – But some say you should use homozygous plants to be sure effect is mutation and not natural variation • Haploid plants seem a reasonable alternative if possible – Very large populations are required to identify desired mutation: • Can you afford to identify marginal traits with replicates & statistics? Estimate: ~10,000 plants for single gene mutant • Clear Objective Embryo Culture Uses • Rescuing interspecific and intergeneric hybrids – wide hybrids often suffer from early spontaneous abortion – cause is embryo-endosperm failure – Gossypium, Brassica, Linum, Lilium • Production of monoploids – useful for obtaining "haploids" of barley, wheat, other cereals – the barley system uses Hordeum bulbosum as a pollen parent Embryo Culture of Citrus Coconut embryo culture Bulbosum Method Hordeum vulgare Barley 2n = 2X = 14 X ↓ Hordeum bulbosum Wild relative 2n = 2X = 14 Embryo Rescue Haploid Barley 2n = X = 7 H. Bulbosum chromosomes eliminated • This was once more efficient than microspore culture in creating haploid barley • Now, with an improved culture media (sucrose replaced by maltose), microspore culture is much more efficient (~2000 plants per 100 anthers) Bulbosum technique Hordeum vulgare is the seed parent zygote develops into an embryo with elimination of Hordeum bulbosum chromosomes eventually, only HV chromosomes are left embryo is "rescued“ to avoid abortion Excision of the immature embryo: Hand pollination of freshly opened flowers Surface sterilization – EtOH on enclosing structures Dissection – dissecting under microscope necessary Plating on solid medium – slanted media are often used to avoid condensation Culture Medium – Mineral salts – K, Ca, N most important – Carbohydrate and osmotic pressure – Amino acids – Plant growth regulators Culture Medium –Carbohydrate and osmotic pressure » » » » 2% sucrose works well for mature embryos 8-12% for immature embryos transfer to progressively lower levels as embryo grows alternative to high sucrose – auxin & cyt PGRs – amino acids » reduced N is often helpful » up to 10 amino acids can be added to replace N salts, incl. glutamine, alanine, arginine, aspartic acid, etc. » requires filter-sterilizing a portion of the medium Culture Medium – natural plant extracts » » » coconut milk (liquid endosperm of coconut) enhanced growth attributed to undefined hormonal factors and/or organic compounds others – extracts of dates, bananas, milk, tomato juice – PGRs » » » » globular embryos – require low conc. of auxin and cytokinin heart-stage and later – usually none required GA and ABA regulate "precocious germination“ GA promotes, ABA suppresses “Wide” crossing of wheat and rye requires embryo rescue and chemical treatment to double the number of chromosomes. Triticale Haploid Plant Production Embryo rescue of interspecific crosses – Creation of alloploids Anther culture/Microspore culture – Culturing of Anthers or Pollen grains (microspores) – Derive a mature plant from a single microspore Ovule culture – Culturing of unfertilized ovules (macrospores) Initiation from Stamens and Pistils Stamen explant Embryogenic callus Callus formation from Callus formation from connective tissue filament tip Embryo development Embryo germination Poliploidization Specific Examples of DH uses • Evaluate fixed progeny from an F1 – Can evaluate for recessive & quantitative traits – Requires very large dihaploid population, since no prior selection – May be effective if you can screen some qualitative traits early • For creating permanent F2 family for molecular marker development • For fixing inbred lines (novel use?) – Create a few dihaploid plants from a new inbred prior to going to Foundation Seed (allows you to uncover unseen off-types) • For eliminating inbreeding depression (theoretical) – If you can select against deleterious genes in culture, and screen very large populations, you may be able to eliminate or reduce inbreeding depression – e.g.: inbreeding depression has been reduced to manageable level in maize through about 50+ years of breeding; this may reduce that time to a few years for a crop like onion or alfalfa Somatic Hybridization Development of hybrid plants through the fusion of somatic protoplasts of two different plant species/varieties Somatic hybridization technique 1. isolation of protoplast 2. Fusion of the protoplasts of desired species/varieties 3. Identification and Selection of somatic hybrid cells 4. Culture of the hybrid cells 5. Regeneration of hybrid plants Isolation of Protoplast (Separartion of 1. Mechanical Method protoplasts from plant tissue) 2. Enzymatic Method Mechanical Method Cells Plasmolysis Plant Tissue Microscope Observation of cells Cutting cell wall with knife Release of protoplasm Collection of protoplasm Mechanical Method Used for vacuolated cells like onion bulb scale, radish and beet root tissues Low yield of protoplast Laborious and tedious process Low protoplast viability Enzymatic Method Leaf sterlization, removal of epidermis Plasmolysed cells Plasmolysed cells Pectinase +cellulase Protoplasm released Pectinase Protoplasm released Release of isolated cells cellulase Isolated Protoplasm Enzymatic Method Used for variety of tissues and organs including leaves, petioles, fruits, roots, coleoptiles, hypocotyls, stem, shoot apices, embryo microspores Mesophyll tissue - most suitable source High yield of protoplast Easy to perform More protoplast viability Protoplast Fusion (Fusion of protoplasts of two different genomes) 1. Spontaneous Fusion Intraspecific Intergeneric 2. Induced Fusion Chemofusion Mechanical Fusion Electrofusion Uses for Protoplast Fusion Combine two complete genomes – Another way to create allopolyploids In vitro fertilization Partial genome transfer – Exchange single or few traits between species – May or may not require ionizing radiation Genetic engineering – Micro-injection, electroporation, Agrobacterium Transfer of organelles – Unique to protoplast fusion – The transfer of mitochondria and/or chloroplasts between species Spontaneous Fusion • Protoplast fuse spontaneously during isolation process mainly due to physical contact • Intraspecific produce homokaryones • Intergeneric have no importance Induced Fusion Chemofusion- fusion induced by chemicals • Types of fusogens • • • • PEG NaNo3 Ca 2+ ions Polyvinyl alcohol Induced Fusion • Mechanical Fusion- Physical fusion of protoplasts under microscope by using micromanipulator and perfusion micropipette • Electrofusion- Fusion induced by electrical stimulation • Fusion of protoplasts is induced by the application of high strength electric field (100kv m-1) for few microsecond Possible Result of Fusion of Two Genetically Different Protoplasts = chloroplast = mitochondria Fusion = nucleus heterokaryon cybrid hybrid hybrid cybrid Identifying Desired Fusions • Complementation selection – Can be done if each parent has a different selectable marker (e.g. antibiotic or herbicide resistance), then the fusion product should have both markers • Fluorescence-activated cell sorters – First label cells with different fluorescent markers; fusion product should have both markers • Mechanical isolation – Tedious, but often works when you start with different cell types • Mass culture – Basically, no selection; just regenerate everything and then screen for desired traits Advantages of somatic hybridization • Production of novel interspecific and intergenic hybrid – Pomato (Hybrid of potato and tomato) • Production of fertile diploids and polypoids from sexually sterile haploids, triploids and aneuploids • Transfer gene for disease resistance, abiotic stress resistance, herbicide resistance and many other quality characters • Production of heterozygous lines in the single species which cannot be propagated by vegetative means • Studies on the fate of plasma genes • Production of unique hybrids of nucleus and cytoplasm Problem and Limitation of Somatic Hybridization 1. Application of protoplast technology requires efficient plant regeneration system. 2. The lack of an efficient selection method for fused product is sometimes a major problem. 3. The end-product after somatic hybridization is often unbalanced. 4. Development of chimaeric calluses in place of hybrids. 5. Somatic hybridization of two diploids leads to the formation of an amphiploids which is generally unfavorable. 6. Regeneration products after somatic hybridization are often variable. 7. It is never certain that a particular characteristic will be expressed. 8. Genetic stability. 9. Sexual reproduction of somatic hybrids. 10. Inter generic recombination. TYPICAL SUSPENSION PROTOPLAST + LEAF PROTOPLAST PEG-INDUCED FUSION NEW SOMATIC HYBRID PLANT True in vitro fertilization A procedure that involves retrieval of eggs and sperm from the male and female and placing them together in a laboratory dish to facilitate fertilization Using single egg and sperm cells and fusing them electrically Fusion products were cultured individually in 'Millicell' inserts in a layer of feeder cells The resulting embryo was cultured to produce a fertile plant Requirements for plant genetic transformation • Trait that is encoded by a single gene • A means of driving expression of the gene in plant cells (Promoters and terminators) • Means of putting the gene into a cell (Vector) • A means of selecting for transformants • Means of getting a whole plant back from the single transformed cell (Regeneration)