* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Plant Tissue Culture:
Hybrid (biology) wikipedia , lookup
Historia Plantarum (Theophrastus) wikipedia , lookup
Ornamental bulbous plant wikipedia , lookup
Cultivated plant taxonomy wikipedia , lookup
Venus flytrap wikipedia , lookup
History of botany wikipedia , lookup
Plant defense against herbivory wikipedia , lookup
Plant use of endophytic fungi in defense wikipedia , lookup
Flowering plant wikipedia , lookup
Plant physiology wikipedia , lookup
Plant secondary metabolism wikipedia , lookup
Sustainable landscaping wikipedia , lookup
Plant breeding wikipedia , lookup
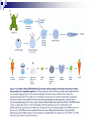
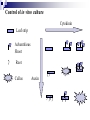
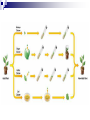
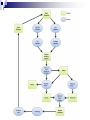
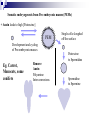
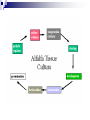
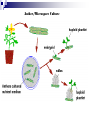
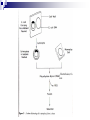
Plant Tissue Culture: History; • Schwann and Schleiden (1838) - Totipotency theory • Haberlandt (1902) - Concept of cell culture published in first report “Experiments on the culture of isolated plant cells” • Skoog and Miller (1957) - Predicted hypothesis “ Regulation of initiation of shoot and root in cultured callus by auxin and cytokinin” • Steward (1958) and Reinert (1959) - Somatic embryogenesis in carrot cultures • Murashige and Skoog (1962) - MS medium • Chilton (1983) - Production of transgenic tobacco • 1984 – onwards - Commercial multiplication of crops, disease free crops, release of GM crops like tomato, soybean, corn, cotton, canola, potato etc. What is plant tissue culture? • It is a collection of techniques used to maintain or grow plant cells, tissues or organs under sterile conditions on a nutrient culture medium of known composition i.e. in vitro • Plant tissue culture is widely used to produce clones of a plant in a method known as micro-propagation • Different techniques in plant tissue culture may offer certain advantages over traditional methods of propagation, including: production of exact copies of plants that produce particularly good flowers, fruits, or have other desirable traits quick production of mature plants production of multiples of plants in the absence of seeds or necessary pollinators to produce seeds regeneration of whole plants from plant cells that have been genetically modified The production of plants in sterile containers that allows them to be moved with greatly reduced chances of transmitting diseases, pests, and pathogens The production of plants from seeds that otherwise have very low chances of germinating and growing i.e. Orchids and Nepenthes (tropical pitcher plants or monkey cups) To clean particular plants of viral and other infections and to quickly multiply these plants as 'cleaned stock' for horticulture and agriculture • Plant tissue culture relies on the fact that many plant cells have the ability to regenerate a whole plant (totipotency) Single cells, plant cells without cell walls (protoplasts), pieces of leaves, stems or roots can often be used to generate a new plant on culture media given the required nutrients and plant hormones Fig 7.2 Alberts 5th Ed Basis for Plant Tissue Culture: • Two hormones affect plant differentiation: Auxin - stimulates root development Cytokinin - stimulates shoot development • Generally, the ratio of these two hormones can determine plant development: auxin ↓cytokinin - root development cytokinin ↓auxin - shoot development auxin = cytokinin - callus development Figs downloaded Factors Affecting Plant Tissue Culture: • Growth Media - Minerals, Growth factors, Carbon source Control of in vitro culture Cytokinin Leaf strip Adventitious Shoot Root Callus Auxin • Environmental Factors - Light, Temperature, Photoperiod • Explant Source - Usually, the younger, less differentiated the explant, the better for tissue culture like ; Shoot tips Axillary buds Seeds Hypocotyl from germinated seed Leaves Fundamental Abilities of Plants: • Totipotency; The potential or inherent capacity of a plant cell to develop into an entire plant if suitably stimulated It implies that all the information necessary for growth and reproduction of the organism is contained in the cell • Differentiation; The capacity of mature cells to return to meristematic condition and development of a new growing point, followed by redifferentiation which is the ability to reorganize into new organs • Competency; The endogenous potential of a given cell or tissue to develop in a particular way Types of in vitro Culture: • Culture of intact plants (seed orchid culture) • Embryo culture (embryo rescue) • Organ culture Shoot tip culture Root culture Leaf culture Anther culture • Callus culture • Cell suspension and single cell culture • Protoplast culture Fig Breeding Applications of Tissue Culture: • Micropropagation • Germplasm preservation • Somaclonal variation • Embryo culture • Haploid and dihaploid production • In vitro hybridization - protoplast fusion • Plant genetic engineering • Micropropagation; Embryogenesis o Direct embryogenesis o Indirect embryogenesis Organogenesis o Organogenesis via callus formation o Direct adventitious organ formation Microcutting o Meristem and shoot tip culture o Bud culture Somatic Embryogenesis: o The process of initiation and development of embryos or embryo-like structures from somatic cells o The production of embryos from somatic or “non-germ” cells o Usually involves a callus intermediate stage which can result in variation among seedlings o Not a common micro-propagation technique but is currently being used to produce superior pine seedlings o Tissue culture maintains the genetic of the cell or tissue used as an explants o Tissue culture conditions can be modified to cause to somatic cells to reprogram into a bipolar structure o These bipolar structures behave like a true embryo - called somatic embryos Organogenesis: o The process of initiation and development of a structure that shows natural organ form and/or function Figs Somatic embryogenesis from Pro-embryonic masses (PEMs) + Auxin leads to high [Putrescine] PEM Single cells sloughed off the surface Development and cycling of Pro-embryonic masses Eg. Carrot, Monocots, some conifers Remove Auxin Polyamine Inter-convesions Putrescine to Spermidine Spermidine to Spermine Secondary embryo formation - Mostly in dicots Abundant Secondary Embryos + Cytokinin Early embryo + Charcoal + ABA - Cytokinin o The ability of non-meristematic plant tissues to form various organs de novo o The production of roots, shoots or leaves o These organs may arise out of pre-existing meristems or out of differentiated cells o This, like embryogenesis, may involve a callus intermediate but often occurs without callus Fig o Somatic embryogenesis and Organogenesis - Both of these technologies can be used as methods of micro-propagation o Not always desirable because they may not always result in populations of identical plants o The most beneficial use of somatic embryogenesis and organogenesis is in the production of whole plants from a single cell or a few cells Tissue Culture Applications: • Micropropagation • Germplasm preservation • Somaclonal variation Tissue culture of Lilium speciosum (source: Chang et al., 2000) Regeneration in Callus Cultures Different stages of embryo formation 4- Stages of embryos Callus Regeneration • Synthetic/Artificial seeds • Embryo culture • Haploid and dihaploid production • In vitro hybridization – protoplast fusion • Industrial products from cell cultures • Plant genetic engineering • Micropropagation: The art and science of plant multiplication in vitro Usually derived from meristems or vegetative buds) without a callus stage Tends to reduce or eliminate somaclonal variation, resulting in true clones Can be derived from other explant or callus but these are often problematic Steps of Micropropagation are: o Stage 0 i.e. selection and preparation of the mother plant sterilization of the plant tissue takes place o o o o Stage I i.e. Initiation of culture - explant placed into growth media Stage II i.e. Multiplication - explants transferred to shoot media; shoots can be constantly divided Stage III i.e. Rooting - explants transferred to root media Stage IV i.e. Transfer to soil - explants returned to soil; hardened off Fig Features of Micropropagation: o Clonal reproduction - Way of maintaining heterozygozity o Multiplication Stage can be recycled many times to produce an unlimited number of clones - Routinely used commercially for many ornamental species, some vegetatively propagated crops o Easy to manipulate production cycles - Not limited by field seasons/environmental influences o Disease-free plants can be produced - Has been used to eliminate viruses from donor plants Fig • Germplasm Preservation: Two methods In Vitro Clonal Propagation of Plants Slow growth techniques; i. ii. o ↓ Temp., ↓ Light, media supplements (osmotic inhibitors, growth retardants), tissue dehydration o Medium-term storage (1 to 4 years) Cryo-preservation; o Ultra low temperatures - liquid nitrogen (-196oC) in presence of Cryoprotective agents like Glycerol, DMSO, PEG o Stops cell division and metabolic processes o Very long-term (indefinite) • Somaclonal Variation: A general phenomenon of all plant regeneration systems that involve a callus phase Two general types of Somaclonal Variation; i. Heritable, genetic changes (alter the DNA) ii. Stable, but non-heritable changes (alter gene expression, epigenetic) Done in cell suspension culture o Then apply physical or chemical mutagen and selection pressure to culture o Regeneration of whole plants from surviving cells Fig o It Important alternative of creation of additional genetic variability in crops where tissue culture and regeneration system has been established - Somaclonal mutants can be enriched during in vitro culture include - Resistance to disease and herbicides - Tolerance to environmental stress and chemical stress - Increased seedling vigor in lettuce - Joint less pedicels in tomato - Increase production of secondary metabolites - New varieties have been developed through somaclonal variation in tomato, sugar cane, celery, Brassica, sorghum • Synthetic / Artificial Seeds; Synthetic or artificial seeds are somatic embryos engineered for use in the commercial propagation of plants These techniques have been further developed for the production of plants from embryos developed by non-sexual methods (haploid production discussed later) Very useful technology where o True seeds are not used or readily available for multiplication (e.g. potato) o hybrid plants (e.g. hybrid rice) o Vegetatively propagated plants are more prone to infections (e.g. day lily, garlic, potato, sugarcane, sweet potato, grape and mango). Synthetic seeds are also useful for multiplication of o Genetically engineered plants (transgenic plants) o Somatic and cytoplasmic hybrids (obtained through protoplast fusion techniques) o Sterile and unstable genotypes o preservation of desirable elite genotypes • Embryo Culture; Embryo culture developed from the need to rescue embryos (embryo rescue) from wide crosses where fertilization occurred, but embryo development did not occur Somatic embryos encapsulated in gel These techniques have been further developed for the production of plants from embryos developed by non-sexual methods (haploid production discussed later) It is use to o rescue F1 hybrid from a wide cross o overcome seed dormancy, usually with addition of hormone to media (GA) o overcome immaturity in seed o speed generations in a breeding program o rescue a cross or self (valuable genotype) from dead or dying plant Fig • Haploid Plant Production; Anther culture/Microspore culture - culturing of anthers or pollen grains (microspores) - derive a mature plant from a single microspore Fig Ovule culture - culturing of unfertilized ovules (macrospores) o Effective for crops that do not yet have an efficient microspore culture system. For example Melon, onion Anther/Microspore Culture Anther Culture What do you do with the haploid? o Weak, sterile plant o Usually want to double the chromosomes, creating a di-haploid plant with normal growth and fertility o Chromosomes can be doubled by a. Colchicine treatment b. Spontaneous doubling a. Tends to occur in all haploids at varying levels b. Many systems rely on it, using visual observation to detect spontaneous di-haploids c. Can be confirmed using flow cytometry • Protoplast Fusion; Figs It is used to o Combine two complete genomes - another way to create allopolyploids , Electrofusion, Ca ions o Partial genome transfer - exchange single or few traits between species which may or may not require ionizing radiation o Genetic engineering Agrobacterium o Transfer of organelles - unique to protoplast fusion i.e. the transfer of mitochondria and/or chloroplasts between species - micro-injection, electroporation, Fig o • Examples are; - Protoplast fusion between male sterile cabbage and normal cabbage was done, and cybrids were selected that contained the radish mitochondria and the cabbage chloroplast - Current procedure is to irradiate the cytoplasmic donor to eliminate nuclear DNA – routinely used in the industry to re-create male sterile brassica crops Industrial Products; Secondary metabolites produced by plants - alkaloids, terpenoids, steroids, anthocyanins, anthraquinones, polyphenols Often restricted production - specific species, tissue or organ • Plant Genetic Engineering Possible Result of Fusion of Two Genetically Different Protoplasts = chloroplast = mitochondria Fusion = nucleus heterokaryon cybrid hybrid hybrid cybrid Over All Applications: • Plant tissue culture is used widely in the plant sciences, forestry, and in horticulture. Applications include: • The commercial production of plants used as potting, landscape, and florist subjects, which uses meristem and shoot culture to produce large numbers of identical individuals • To conserve rare or endangered plant species • A plant breeder may use tissue culture to screen cells rather than plants for advantageous characters, e.g. herbicide resistance/tolerance. • Large-scale growth of plant cells in liquid culture in bioreactors for production of valuable compounds, like plant derived secondary metabolites and recombinant proteins used as biopharmaceuticals • To cross distantly related species by protoplasm fusion and regeneration of the novel hybrid • To rapidly study the molecular basis for physiological, biochemical, and reproductive mechanisms in plants, for example in vitro selection for stress tolerant plants, and in vitro flowering studies. • To cross-pollinate distantly related species and then tissue culture the resulting embryo which would otherwise normally die (Embryo Rescue) • For chromosome doubling and induction of polypoidy. For example doubled haploids, tetraploids, and other forms of polypoloids. This is usually achieved by application of antimitotic agents such as colchicine or oryzalin • As a tissue for transformation, followed by either short-term testing of genetic constructs or regeneration of transgenic plants • Certain techniques such as meristem tip culture can be used to produce clean plant material from viruses stock, such as potatoes and many species of soft fruit • Production of identical sterile hybrid species can be obtained Cryopreservation Requirements: • Pre-culturing Usually a rapid growth rate to create cells with small vacuoles and low water content • Cryo-protection Glycerol, DMSO, PEG, to protect against ice damage and alter the form of ice crystals • Freezing The most critical phase - one of two methods: - Slow freezing allows for cytoplasmic dehydration - Quick freezing results in fast intercellular freezing with little dehydration • Storage Usually in liquid nitrogen (-196oC) to avoid changes in ice crystals that occur above -100oC • Thawing Usually rapid thawing to avoid damage from ice crystal growth • Recovery Thawed cells must be washed of cryo-protectants and nursed back to normal growth Avoid callus production to maintain genetic stability x ------------------------- x ----------------------- x --------------------------- x