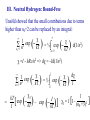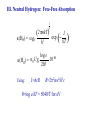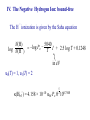* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Spectroscopy – Lecture 1
Energetic neutral atom wikipedia , lookup
Nucleosynthesis wikipedia , lookup
Circular dichroism wikipedia , lookup
Standard solar model wikipedia , lookup
Microplasma wikipedia , lookup
Main sequence wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Stellar evolution wikipedia , lookup
Spectroscopy – Continuous Opacities I. Introduction: Atomic Absorption Coefficents II. Corrections for Stimulated Emission III. Hydrogen IV. Negative Hydrogen Ion V. Negative Helium Ion VI. Metals VII. Electron Scattering VIII. Others IX. Summary In order to calculate the transer of radiation through a model stellar atmosphere, we need to know the continuous absorption coefficient, kn. This shapes the continous spectrum → more absorption, less light. It also influences the strength of stellar lines → more continous absorption means a thinner photosphere with few atoms to make spectral lines. Also, before we compute a theoretical spectrum, you need to compute an atmospheric model, and this also depends on kn. Recall the radiative transfer equation: dIn = –In + jn/kn = –In + Sn dtn To solve this you need to know the opacity. You can have a nice solution, but it will not reproduce observations. The grey atmosphere has a simple opacity, but no bearing with reality. The problem is that there are a lot of opacity sources which are temperature dependent B1I V F0 V G2 V I. The atomic absorption coefficent The total continuous absorption coefficient is the sum of absorption resulting from many physical processes. These are in two categories: • bound-free transition: ionization • free-free transition: acceleration of a charge when passing another charge bound-bound transitions result in a spectral line and are not included in kn but in cool stars line density is so great it affects the continuum I. The atomic absorption coefficent The atomic absorption coefficient, a, has units of area per absorber. The wavelength versus frequency question does not arise for a: an = al a is not a distribution like In and Il, but the power subtracted from In in interval dn is adn. This is a distribution and has units of erg/(s cm2 rad2 Hz) a dn =(c/l2) a dl II. Corrections for Stimulated Emission Recall that the stimulated emission (negative absorption) reduces the absorption: knr = NℓBℓuhn – NuBuℓhn = Nℓ Bℓuhn(1– NuBuℓ/NℓBℓu) = NℓBℓuhn[1– exp(–hn/kT)] Nu Blu Nℓ Bul lT cm K T 0.2 0.4 0.6 0.8 1.0 4000 8000 12000 16000 20000 Teff 30000 3000 1– exp(hn/kT) % decrease 0.999 0.973 0.909 0.834 0.763 l (10% reduction) 2500 Ang 2mm 0.08 2.8 10.0 19.8 31.1 13.60 12.75 12.08 Brackett Pachen Balmer Lyman III. Atomic Absorption Coefficient for Hydrogen ½ mv2 = hn–hRc/n2 R = 1.0968×105 cm–1 ·· n=∞ n=4 n=3 Ha b 10.20 0.00 c = 13.6(1–1/n2) eV n=2 n=1 ½ mv2 = hn–hRc/n2 R = 1.0968×105 cm–1 At the ionization limit v=0, n=Rc/n2 Absorption edges: n l Ang 1 912 Lyman 2 3647 Balmer 3 8206 Paschen 4 14588 Brackett 5 22790 Pfund Name III. Neutral Hydrogen: Bound-Free Original derivation is from Kramers (1923) and modified by Gaunt (1930): pe6 R g ∕ an = 6.16 n 5 3 h nn an = 6.16 p2e6 h3c3 R l3 Per neutral H atom gn∕ = a0 gn∕ l3 n5 n5 a0 = 1.044×10–26 for l in angstroms e = electron charge = 4.803×10–10 esu gn∕ = Gaunt factor needed to make Kramer´s result in agreement with quantum mechanical results n =1 n =2 n =3 n=4 n=5 a0, 10×–17 cm2/H atom n=6 ~l3/n5 Wavelength (Angstroms) ~l3 n=7 III. Neutral Hydrogen: Bound-Free The sum of absorbers in each level times an is what is needed. Recall: Nn N gn c exp – = u0(T) kT ( ) gn=2n2 u0(T) = 2 c =I – hRc/n2 = 13.6(1-1/n2) eV III. Neutral Hydrogen: Bound-Free The absorption coefficient in square centimeters per neutral hydrogen atom for all continua starting at n0 ∞ k(Hbf) = Σ n 0 anNn N = a0 = a0 ∞ Σ n0 ∞ Σ n0 l3 ∕ c g n3 n exp – kT ( l3 ∕ gn 10–qc 3 n q = 5040/T, c in electron volts ) III. Neutral Hydrogen: Bound-Free Unsöld showed that the small contributions due to terms higher than n0+2 can be replaced by an integral: ∞ Σ n0+3 1 exp – c n3 kT ( ) ∞ c = ½ ∫ exp – kT n +3 ( 0 ) d(1/n2) c =I – hRc/n2 => dc = –Id(1/n2) ∞ Σ n0+3 = 1 exp – c n3 kT ( kT exp – c3 [ kT I ( )– I ) = ½∫ c3 exp c exp – kT I ( (– kT) ] ) dc I 1 c3 = I [1– (n +3)2 ] 0 III. Neutral Hydrogen: Bound-Free We can neglect the n dependence on gn∕ and the final answer is: k(Hbf) = a0l3 n0+2 [Σ n0 gn∕ –qc log e –c q –Iq) (10 – 10 + 3 ] 2qI n3 10 This is the bound free absorption coefficient for neutral hydrogen n =1 n =2 n =3 n=4 n=5 n=6 n=7 III. Neutral Hydrogen: Bound-Free = kbf(n=3) + ... kbf(n=2) + kbf(n=3) + ... ≈ kbf(n=3) kbf(n=2) 8 = 27 exp [ –(c3 – c2)/kT [ kbf(>3647) kbf(<3647) = 0.0037 at 5000 K and 0.033 at 10000 K III. Neutral Hydrogen: Bound-Free k(red side)/k(blue side) l edge (Ang) T 3000 T 5000 T 10000 T 30000 Lyman 912 9×10–19 6×10–12 9×10–7 0.002 Balmer 3647 0.00021 0.00376 0.033 0.14 Paschen 8206 0.03 0.089 0.177 0.31 Brackett 14588 0.16 0.255 0.36 0.45 Pfund 0.30 0.39 0.48 0.54 22790 III. Neutral Hydrogen: Bound-Free III. Optical Depth and Height of Formation 3647 8602 continuum Flux 912 Across a jump your are seeing very different heights in the atmosphere Wavelength Recall: tn = knrdx tn ~ 2/3 for Grey atmosphere As an increases, kn increases => dx decreases You are looking higher in the atmosphere Temperature profile of photosphere Temperature k(<3647) > k(>3647) => dx2 > dx1 l<3647 A l>3647 A 10000 8000 6000 4000 z=0 z z=0 dx1 dx2 t=2/3 t=2/3 z B4 V But wait, I just said that the Balmer jump should be larger for cooler stars. Why is this not the case? For cooler stars other sources of opacity start to dominate, namely H– Maximum of black body = lT = 0.5099 cm K But peak implies T=13400 K But peak implies T=13400 K The stronger opacity of on the blue side of the Balmer jump distorts the Planck curve. One cannot use the peak of the intensity, but must fit the full spectral energy distribution Amplitude (mmag) Photometric Amplitude of rapidly oscillating Ap stars: Wavelength (Ang) Different wavelengths probe different heights in atmosphere III. Neutral Hydrogen: Free-Free The free-free absorption of hydrogen is much smaller. When the free electron has a collision with a proton its unbound orbit is altered. The electron can absorbs a photon and its energy increases. The strength of this absorption depends on the velocity of the electron III. Neutral Hydrogen: Free-Free proton e– Orbit is altered The absorption of the photon is during the interaction III. Neutral Hydrogen: Free-Free Absorption According to Kramers the atomic coefficient is: daff h2e2 R 1 = 0.385 pm3 n3 v dv This is the cross section in square cm per H atom for the fraction of the electrons in the velocity interval v to v + dv. To get complete f-f absorption must integrate over v. III. Neutral Hydrogen: Free-Free Absorption Using the Maxwell-Boltzmann distribution for v h2e2 R 1 aff = 0.385 pm3 n3 ∞ 1 2 m kT 3 2 ∫ (p ) ( ) 2 0 h2e2 R 1 aff = 0.385 pm3 n3 2 mv v exp – dv 2kT ( 1 2 ( ) 2m pkT Quantum mechanical derivation by Gaunt is modified by f-f Gaunt factor gf ) III. Neutral Hydrogen: Free-Free Absorption The absorption coefficient in square cm per neutral H atom is proportional to the number density of electrons, Ne and protons Ni: k(Hff) = afgfNiNe N0 Density of neutral H Recall the Saha Equation: 3 2 Ni N Pe = Pe = NekT ( 2pm) ( kT) h3 5 2 I 2u1(T) exp – kT u0(T) ( ) III. Neutral Hydrogen: Free-Free Absorption 3 2 k(Hff) = afgf (2pmkT) k(Hff) = a0l3gf Using: I=hcR h3 I exp – kT ( log e 10–qI 2qI R=2p2me4/h3c q=log e/kT = 5040/T for eV ) III. Total Absorption Coefficient for Hydrogen total bound-free free-free IV. The Negative Hydrogen Ion The hydrogen atom is capable of holding a second electron in a bound state. The ionization of the extra electron requires 0.754 eV All photons with l < 16444 Ang have sufficient – energy to ionize H back to neutral H Very important opacity for Teff < 6000 K Where does this extra electron come from? Metals! IV. The Negative Hydrogen Ion For Teff > 6000 K, H– too frequently ionized to be an effective absorber For Teff < 6000 K, H– very important For Teff < 4000 K, no longer effective because there are no more free electrons IV. The Negative Hydrogen Ion The bound free absorption coefficient can be expressed by the following polynomial abf = a0 + a1l + a2l2 + a3l3 + a4l4 + a5l5 + a6l6 a0 = 1.99654 a1 = –1.18267 × 10–5 a2 = 2.64243 × 10–6 a3 = –4.40524 × 10–10 a4 = 3.23992× 10–14 a5 = –1.39568 × 10–10 a6 = 2.78701 × 10–23 l is in Angstroms abf, 10–18 cm2 per H– ion IV. The Negative Hydrogen Ion: Bound-Free Wavelength (angstroms) IV. The Negative Hydrogen Ion: bound-free – The H ionization is given by the Saha equation N(H) 5040 – –log P e log N(H –) = T I + 2.5 log T + 0.1248 in eV u0(T) = 1, u1(T) = 2 – 5 2 k(Hbf ) = 4.158 × 10–10 abf Pe q 100.754q IV. The Negative Hydrogen Ion: free-free k(Hff = Peaff = –) 10–26 × Pe 10 f0+f1logq+f2log2q f0 = –2.2763–1.685 logl+0.766 log2l–0.0533464 log3l f1 = 15.2827–9.2846 logl+1.99381 log2l–0.142631 log3l f3 = –197.789+190.266 logl–67.9775 log2l+10.6913 log3l–0.625151 log4l IV. The Negative Hydrogen Ion: Total bound-free free-free V. The Negative Helium ion The bound–free absorption is neglible, but free-free can be important in the atmospheres of cool stars and at longer wavelengths VI. Metals • In the visible a minor opacity source because they are not many around • Contribute indirectly by providing electrons • In the visible kn(metals) ~ 1% kn(Hbf–) • A different story in the ultraviolet where the opacity is dominated by metals VI. Metals The absorption coefficient for metals dominate in the ultraviolet VII. Electron (Thompson) Scattering • Important in hot stars where H is ionized • Only true „grey“ opacity source since it does not depend on wavelength • Phase function for scattering ~ 1 + cos q • Stellar atmosphere people assume average phase ~ 0 VII. Electron (Thompson) Scattering The absorption coefficient is wavelength independent: ( ( 8p ae = 3 e2 2 –24 cm2/electron = 0.6648 x 10 2 mc The absorption per hydrogen atom: k(e) = aeNe NH = aePe PH PH = Partial pressure of Hydrogen VII. Electron (Thompson) Scattering PH is related to the gas and electron pressure as follows: N = S Nj + Ne = NH S Aj + Ne Nj particles of the jth element per cubic cm and Aj = Nj/NH Solving for NH NH = N–Ne S Aj PH = Pg–Pe S Aj VII. Electron (Thompson) Scattering k(e) = aePe S Aj Pg– Pe Electron scattering is important in O and Early B stars If hydrogen dominates their composition Pe = 0.5Pg k(e) = ae S Aj Independent of pressure PTot = Pe + Pg Pe /PTot 0.5 Teff VIII. Other Sources of Opacity H2 molecules • H2 (neutral) has no significant absorption in the visible • H2+, H2– do have significant absorption • H2+(bf) important in the ultraviolet, in A-type stars it is ~ 10% of H– bound-free opacity • Peaks in opacity around l ≈ 1100 Å, is dominated by the Balmer continuum below 3600 Å in most stars • H2– (free-free) important in the infrared (cool stars) and fills the opacity minimum of H– at 16400 Å VIII. Other Sources of Opacity He I (bound-free), He II (bound-free) • Important only in O and B-type stars • He II (bound-free) is hydrogenic → multiply hydrogen cross sections by Z4 or 16. VIII. Other Sources of Opacity Rayleigh Scattering • Important in cool stars • Scattering by molecules and atoms • Has a 1/l4 dependence VIII. Other Sources of Opacity Cool Stars • Molecules and ions: CN–, C2–, H2, He, N2, O2, TiO,.... Basically Cool Stars are a mess and only for the bravest theoretical astrophysicist IX. Summary of Continuous Opacities Spectral Type Dominant opacity O–B Electron scattering, He I,II (b-f), H(f-f) B–A H I: b-f, f-f He II: b-f, some electron scattering A–F equal contributions from H I (b-b) H I (f-f), H– (b-f, f-f) G–K H I (b-f), H– (b-f, f-f), Rayleigh Scattering off H I IX. Summary of Continuous Opacities Spectral Type Dominant opacity K–Early M H– (b-f, f-f), Rayleigh scattering (UV) off H I and H2, molecular opacities (line blanketing) M: Molecules and neutral atoms, H– (b-f, f-f), Rayleigh scattering off other molecules Changes in the continuous opacity is the cause of most, if not all pulsating stars k Mechanism: If in a region of the star the opacity changes, then the star can block energy (photons) which can be subsequently released in a later phase of the pulsation. Helium and and Hydrogen ionization zones of the star are normally where this works. Consider the Helium ionization zone in the interior of a star. During a contraction phase of the pulsations the density increases causing He II to recombine. Neutral helium has a higher opacity and blocks photons and thus stores energy. When the star expands the density decreases and neutral helium is ionized by the emerging radiation. The opacity then decreases. Cepheid Pulsations are due to an opacity effect: Contraction Expansion He II/He III ionization zone During compression He II ionized to He III, He III has a higher opacity. This blocks radiation causing star to expand During expansion He zone cools, He III recombines, opacity decreases allowing photons to escape. Star then contracts under gravity. Most pulsating stars can be explained by opacity effects The End….our only friend the End