* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download April 2005
Canis Minor wikipedia , lookup
Aquarius (constellation) wikipedia , lookup
International Ultraviolet Explorer wikipedia , lookup
Constellation wikipedia , lookup
Corvus (constellation) wikipedia , lookup
H II region wikipedia , lookup
Star formation wikipedia , lookup
Timeline of astronomy wikipedia , lookup
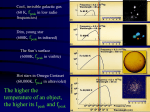
Welcome to Starry Monday at Otterbein Astronomy Lecture Series -every first Monday of the monthApril 4, 2005 Dr. Uwe Trittmann Today’s Topics • Spectra – Fingerprints of the Elements • The Night Sky in March Feedback! • Please write down suggestions/your interests on the note pads provided • If you would like to hear from us, please leave your email / address • To learn more about astronomy and physics at Otterbein, please visit – http://www.otterbein.edu/dept/PHYS/weitkamp.asp (Obs.) – http://www.otterbein.edu/dept/PHYS/ (Physics Dept.) Light and Spectra • Color of light determined by its wavelength • White (visible) light is a mixture of all colors • Can separate individual colors with a prism Light is an electromagnetic Wave • Medium = electric and magnetic field • Speed = 3 105 km/sec Electromagnetic Spectrum Visible Light 400–440 nm Violet 440–480 nm Blue 480–530 nm Green 530–590 nm Yellow 590–630 nm Orange 630–700 nm Red Three Things Light Tells Us • Temperature – from black body spectrum • Chemical composition – from spectral lines • Radial velocity – from Doppler shift Peak frequency Black Body Spectrum (gives away the temperature) • All objects - even you emit radiation of all frequencies, but with different intensities Cool, invisible galactic gas (60 K, mostly low radio frequency) Dim, young star (600K, mostly infrared) The Sun’s surface (6000K, mostly visible) Hot stars in Omega Centauri (60,000K, mostly ultraviolet) The hotter the object, the higher the peak frequency! Wien’s Law • The peak of the intensity curve will move with temperature, this is Wien’s law: Temperature / frequency = constant So: the higher the temperature T, the smaller the frequency f, i.e. the higher the energy of the electromagnetic wave Measuring Temperatures • Find maximal intensity Temperature (Wien’s law) Identify spectral lines of ionized elements Temperature Spectral Lines – Fingerprints of the Elements • Can use this to identify elements on distant objects! • Different elements yield different emission spectra Origin of Spectral Lines • Atoms: electrons orbiting nuclei • Chemistry deals only with electron orbits (electron exchange glues atoms together to from molecules) • Nuclear power comes from the nucleus • Nuclei are very small – If electrons would orbit the statehouse on I-270, the nucleus would be a soccer ball in Gov. Bob Taft’s office – Nuclei: made out of protons (el. positive) and neutrons (neutral) • The energy of the electron depends on orbit • When an electron jumps from one orbital to another, it emits (emission line) or absorbs (absorption line) a photon of a certain energy • The frequency of emitted or absorbed photon is related to its energy E=hf (h is called Planck’s constant, f is frequency) Origin of Spectral Lines: Emission Heated Gas emits light at specific frequencies “the positive fingerprints of the elements” Origin of Spectral Lines: Absorption Cool gas absorbs light at specific frequencies “the negative fingerprints of the elements” Spectral Lines 1. Light of a low density hot gas consists of a series of discrete bright emission lines: the positive “fingerprints” of its chemical elements! 2. A cool, thin gas absorbs certain wavelengths from a continuous spectrum dark absorption ( “Fraunhofer”) lines in continuous spectrum: negative “fingerprints” of its chemical elements, precisely at the same wavelengths as emission lines. Doppler Shift Application: Separate close Binary Stars • Too distant to resolve the individual stars • Can be viewed indirectly by observing the back-and-forth Doppler shifts of their spectral lines Application:Classification of the Stars Class O B A F G K M Temperature 30,000 K 20,000 K 10,000 K 8,000 K 6,000 K 4,000 K 3,000 K Color blue bluish white white yellow orange red Examples Rigel Vega, Sirius Canopus Sun, Centauri Arcturus Betelgeuse Mnemotechnique: Oh, Be A Fine Girl/Guy, Kiss Me The HertzprungRussell Diagram • A plot of absolute luminosity (vertical scale) against spectral type or temperature (horizontal scale) • Most stars (90%) lie in a band known as the Main Sequence Hertzsprung-Russell diagrams … of the closest stars …of the brightest stars Stellar Lifetimes • From the luminosity, we can determine the rate of energy release, and thus rate of fuel consumption • Given the mass (amount of fuel to burn) we can obtain the lifetime • Large hot blue stars: ~ 20 million years • The Sun: 10 billion years • Small cool red dwarfs: trillions of years The hotter, the shorter the life! The Night Sky in March • The sun is getting higher -> shorter nights! • Spring constellations (Cancer,Leo,Coma,Virgo,…) contain few bright stars but many galaxies • Jupiter is in opposition this month (i.e. at its brightest) Moon Phases • Today (Waning crescent, 20%) • 4 / 8 (New Moon) • 4 / 16 (First Quarter Moon) • 4 / 24 (Full Moon) • 5 / 1 (Last Quarter Moon) Today at Noon Sun at meridian, i.e. exactly south 10 PM Typical observing hour, early March no Moon Jupiter Saturn at meridian SouthEast Perseus and Auriga with Plejades and the Double Cluster Zenith Big Dipper points to the north pole SouthWest The Winter Constellations – – – – – Orion Taurus Canis Major Gemini Canis Minor South Spring Constellations - Cancer - Leo - Hydra Deep Sky Objects: - Beehive Cluster (M44) Mark your Calendars! • Next Starry Monday at Otterbein: May 2, 2005, 7 pm (this is a Monday • Web pages: – http://www.otterbein.edu/dept/PHYS/weitkamp.asp (Obs.) – http://www.otterbein.edu/dept/PHYS/ (Physics Dept.) ) Mark your Calendars II • • • • Physics Coffee is every Wednesday, 3:30 pm Open to the public, everyone welcome! Location: across the hall, Science 256 Free coffee, cookies, etc. • Details about Otterbein’s Rocket Contest there!