* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Antrectomy
Survey
Document related concepts
Transcript
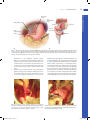
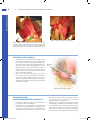
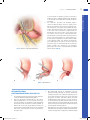
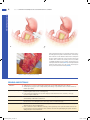
Chapter 10 Antrectomy J. Spencer Liles John D. Christein DEFINITION By strict definition, antrectomy refers to removal of the gastrin-secreting portion of the stomach and when combined with a vagotomy results in an 85% reduction in gastric acid secretion.1,2 In the 1960s and 1970s, antrectomy with or without vagotomy was routinely performed for treatment of benign gastric and duodenal ulcers but, due to pharmacologic developments in reducing acid secretion and elucidation of the role of Helicobacter pylori in ulcer development, is now rarely performed for ulcer disease.3,4 n Today, the term antrectomy is loosely applied to any distal gastric resection and is indicated in recurrent or persistent gastric ulcers to rule out malignancy, complicated peptic ulcer disease (i.e., obstruction, hemorrhage, perforation), and for resection of certain neoplasms of the antrum and pyloric channel (Table 1).3 n When an antrectomy is performed for complicated peptic ulcer disease, a vagotomy may be included to reduce the chance of anastomotic ulcer formation in patients who are not candidates for H. pylori treatment and lifelong proton pump inhibitor therapy due to unreliability, noncompliance, or medication side effects.5,6 n Antrectomy is named by the type of gastrointestinal (GI) anastomosis performed. n Billroth I procedure—antrectomy and gastroduodenostomy n Billroth II procedure—antrectomy and gastrojejunostomy n A modification of the Billroth II procedure that involves a gastrojejunostomy via a Roux limb and is known as a Roux-en-Y gastrojejunostomy n DIFFERENTIAL DIAGNOSIS Complicated peptic ulcer disease and distal gastric neoplasms, both benign and malignant, account for the vast majority of the antral resections performed today. These diagnoses will be discussed separately. n Peptic ulcer disease n Peptic ulcer disease refers to irritation of GI mucosa from gastric acid due to either increased acid presence or n Table 1: Indications for Antrectomy Peptic ulcer disease • Intractability—persistent or recurrent gastric ulcers in order to rule out malignancy • Perforation—distal gastric or duodenal ulcer • Bleeding—type I, II, or III gastric ulcer • Obstruction—any gastric outlet or duodenal obstruction due to chronic ulcer scarring Neoplasm • Benign—single giant gastric polyp or multiple gastric polyps not amenable to endoscopic resection, leiomyoma, lipoma • Malignant—leiomyosarcoma, gastrointestinal stromal tumor, early-stage adenocarcinoma, neuroendocrine tumor • Gastroparesis—role is debated in chronic gastroparesis only after extensive workup eakness in the mucosal protection and typically presents w with epigastric pain.2,4 Peptic ulcers can occur anywhere in the GI tract, but duodenal and gastric ulcers are most common. Duodenal ulcers typically arise within 2 cm of the pylorus, are highly associated with H. pylori infection (.90%), and frequently resolve with appropriate H. pylori therapy. Gastric ulcers are less likely to be associated with H. pylori infection and are classified into five types based on their location and association with acid secretion (Table 2).7 n The differential diagnosis of epigastric pain similar to that found in complicated peptic ulcer disease is chronic cholecystitis, acute pancreatitis, chronic pancreatitis, functional indigestion or dyspepsia, gastritis, and reflux esophagitis. Complicated peptic ulcer disease can also present with upper GI hemorrhage, and a differential should include esophagitis (reflux and infectious); gastroesophageal varices, arteriovenous malformations; Mallory-Weiss tear; stress gastritis; and neoplasm of the esophagus, stomach, duodenum, pancreas, and biliary tree. n Lastly, pyloric obstruction due to chronic inflammation and scarring will cause nausea, emesis, and early satiety. The differential for these symptoms includes gastric motility disorders (i.e., gastroparesis), gastroenteritis, small bowel obstruction, electrolyte abnormalities, and extrinsic compression from pancreatic pseudocysts or neoplasms. n Distal gastric neoplasms—Gastric neoplasms include benign polyps, adenocarcinoma, neuroendocrine tumors, lymphoma, B-cell mucosa-associated lymphoid tissue (MALT) lymphomas, GI stromal tumors, leiomyomas, and leiomyosarcomas. Any gastric neoplastic process can cause upper GI bleeding, epigastric pain, and luminal obstruction, and a differential similar to peptic ulcer disease should be considered. PATIENT HISTORY AND PHYSICAL FINDINGS All patients should undergo a thorough history and physical exam with questions focusing on the nature of the symptoms, specifically determining the relationship between symptoms and eating, deciphering whether symptoms are acute or chronic, and determining the severity of the symptoms. A vast majority of patients will have abdominal pain. Pain related to peptic ulcer disease that results from the corrosive effect of gastric acid on vulnerable GI mucosa and typically occurs in the epigastrium is described as gnawing or burning and follows a daily cycle. This pain typically arises shortly after eating breakfast and persists until lunch at which time the oral intake alleviates the pain. Relief is transient and pain recurs in the early afternoon and again persists until dinner. Meals, specifically ones consisting of milk and dairy products, and antacids provide temporary relief from ulcer pain. Acute, severe epigastric pain can signify ulcer perforation, whereas back pain suggests ulcer penetration into the pancreas.8 n 1 Mulholland_Part2_Ch10.indd 1 1/24/14 5:18 PM 2 P a r t 2 OPERATIVE TECHNIQUES IN GASTROINTESTINAL SURGERY Table 2: Peptic Ulcer Disease Location Common Complications Acid Secretion Gastroesophageal junction and distal esophagus Hemorrhage High I Gastric body on lesser curvature near the angularis incisura Perforation Normal or low • Older patients • Associated with Helicobacter pylori II Two ulcers; gastric body and duodenal ulcer Hemorrhage, obstruction, or perforation High • Younger patients • Association with active or quiescent duodenal ulcers III Prepyloric Hemorrhage, perforation High • Younger patients • Similar to type II gastric ulcers and duodenal ulcers IV High on lesser curvature Hemorrhage Low • Likely a variant of type I gastric ulcers • Difficult to treat surgically V Anywhere Perforation Normal • Related to NSAID use 95% occur within 2 cm of pylorus Hemorrhage, obstruction, or perforation Normal or high Esophageal Other Gastric Duodenal NSAID, nonsteroidal antiinflammatory drug. Nausea and vomiting can be seen with ulcer disease even in the absence of pyloric obstruction. Nausea that is chronic in nature and associated with early satiety and weight loss suggests inflammation and scarring of the pyloric channel due to chronic ulceration. n It is not uncommon for complicated peptic ulcers to present with upper GI bleeding, perforation, or obstruction in a patient with no history of peptic ulcer disease. n Bleeding—hematemesis, melena, recent diagnosis of anemia n Perforation—acute onset upper abdominal pain and peritonitis n Obstruction—nausea, emesis, food regurgitation, early satiety, weight loss n Mulholland_Part2_Ch10.indd 2 Acute or chronic upper GI bleeding can signify complicated ulcer disease and may present as melena, weakness, fatigue, general malaise, or a recent diagnosis of anemia. n Risk factors for developing ulcer disease include a history of H. pylori infection; smoking; Zollinger-Ellison syndrome; and use of nonsteroidal antiinflammatory drugs (NSAIDs), steroids, and other immunosuppressive medications.1,8 Therefore, an accurate medication list should be obtained and reviewed with the patient. History of previous ulcer disease should be elicited, and the success and timing of previous treatment modalities should be documented. Presence of H. pylori infection, completion of antibiotic therapy, and documentation of eradication is crucial (Table 3). Ulcers that persist despite appropriate n 1/24/14 5:18 PM C h a p t e r 10 ANTRECTOMY 3 Table 3: Helicobacter pylori Testing Sensitivity Specificity .95% .90% Fecal antigen detection Serology Invasive (endoscopic) Biopsy urease test .90% 85% .95% 79% .90% .95% Histology Culture .90% 80% .95% 100% Noninvasive Urease breath test Other Determines active infection but must stop PPI 2 wk prior; takes 30–60 min and is used to confirm eradication Determines active infection but is positive for up to 12 wk after eradication Cannot be used to confirm eradication because antibodies persist Decreased sensitivity with ongoing PPI, H2 antagonist, antibiotic, and bismuth compound use or with recent GI bleeding Multiple available stains and widely used; increased sensitivity with increased biopsies Difficult and expensive; reserved for persistent infections and antibiotic sensitivity testing PPI, proton pump inhibitor; GI, gastrointestinal. t reatment of H. pylori, cessation of NSAID use, or are found in H. pylori–negative patients should raise suspicion for underlying malignancy. n A gastric lesion can also present with epigastric pain and obstruction. This pain is typically vaguer in nature and lacks a gnawing or burning component. Furthermore, these patients may describe a sensation of persistent fullness and early satiety despite hunger. n A subjective assessment of nutrition and functional status is necessary to evaluate the patient’s ability to tolerate a major surgical procedure. IMAGING AND OTHER DIAGNOSTIC STUDIES Diagnostic evaluation of suspected peptic ulceration and upper GI lesions can include endoscopy, contrast radiography, and computed tomography (CT) (FIG 1). n Endoscopy is felt to be superior because it allows for tissue sampling. Sampling of gastric mucosa can be used to confirm the presence of H. pylori. On endoscopy, ulcers are sharply demarcated; often have exposed underlying submucosa; and frequently occur in the first portion of the duodenum, the prepyloric area, and the pyloric channel. All gastric ulcers n should be biopsied at least six times at the ulcer edge and brush biopsied to evaluate for underlying malignancy. Even if negative for malignancy, repeat endoscopy after medical treatment is indicated to evaluate for therapeutic response, and in instances of persistent or intractable disease, ulcer resection is indicated.8 n Other relevant imaging modalities include contrast radiography and CT. Double-contrast radiography of the upper GI tract detects roughly 90% of gastric and duodenal ulcers but does not allow for tissue sampling. In the acute setting, CT is helpful in identifying gastric or duodenal ulcer perforation. Additionally, CT can identify wall thickening in chronic ulcer inflammation or neoplastic situations but again lacks the ability to provide tissue sampling. n Zollinger-Ellison syndrome is a rare condition of increased serum gastrin levels secondary to a gastrinoma with resulting severe ulcer disease. In nonsmoking patients who are negative for H. pylori, serum fasting gastrin levels should be obtained to evaluate for Zollinger-Ellison syndrome. Normal basal gastrin levels average to 50 to 100 pg/mL, and levels over 200 pg/mL can almost always be considered high. Diagnosis of ZollingerEllison syndrome can alter your treatment approach.9 SURGICAL MANAGEMENT The indications for antrectomy are listed in Table 1. As explained earlier, an antrectomy is rarely performed for its original purpose of removing the antrum and reducing acid secretion. n Antrectomy is not the primary treatment option for bleeding or perforated peptic ulcers. A vast majority of bleeding ulcers is controlled endoscopically and, in the 5% to 10% that require operative intervention, a formal antrectomy is rarely needed. Roughly 90% of perforated ulcers can be safely controlled with primary closure and omental patching. Thus, antrectomy for bleeding or perforated ulcers is reserved for cases when less invasive treatment options are ineffective. n B A C FIG 1 l Radiology. A. CT scan of pyloric obstruction from chronic pyloric inflammation. B. Endoscopic image of near-complete pyloric obstruction from chronic pyloric inflammation (left); endoscopic image of a large gastric ulcer with a central necrotic region (right). C. Contrast radiography demonstrating pyloric obstruction from chronic pyloric inflammation. Mulholland_Part2_Ch10.indd 3 Preoperative Planning n All patients should undergo preoperative endoscopy to identify the extent of disease and preoperative nutritional assessment. All patients should receive preoperative antibiotics in a timely fashion to reduce the risks of perioperative infectious complications from gram-positive cocci and enteric gram-negative bacilli pathogens. 1/24/14 5:18 PM 4 P a r t 2 OPERATIVE TECHNIQUES IN GASTROINTESTINAL SURGERY Several factors must be considered when deciding between performing a Billroth I and Billroth II procedure. The advantage to a Billroth I procedure is that the anatomic arrangement of the GI tract is preserved, which maintains the innate regulatory pathways of bicarbonate and pancreatic enzymes and significantly decreases the rate of postprandial dumping. Unfortunately, the lack of a pylorus results in bile reflux gastritis in a majority of patients. A Billroth I procedure cannot always be performed due to inflammation and scarring from prepyloric, pyloric, or duodenal ulcers. In these instances, the Billroth II procedure allows for a tension-free anastomosis of noninflamed tissue but introduces the problems of potential afferent loop syndrome and bile reflux gastritis, whereas a Roux-en-Y gastrojejunostomy diminishes the occurrence of bile reflux at the cost of a second anastomosis. Lastly, in cases of invasive neoplasms or concerning gastric masses, a Billroth II procedure with or without reconstruction with a Roux-en-Y gastrojejunostomy is preferred as it allows for dissection of much wider margins and is less likely to obstruct in the unfortunate setting of recurrent disease.10,11 TECHNIQUES n Positioning The patient should be positioned supine with arms out. A urinary catheter and a nasogastric tube should be placed to decompress the stomach. Positioning should allow for attachment of a self-retaining retractor system to the operating room table. n EXPOSURE n and a self-retaining retractor system is placed to widely expose the upper abdomen (FIG 2). A midline supraumbilical incision is made and carried to the level of the xiphoid. The falciform ligament is divided A B FIG 2 l A. A midline upper abdominal incision is used. B. Division of the falciform ligament and placement of self-retaining retractor allows adequate exposure of the upper abdomen. GASTRIC MOBILIZATION n Mobilization of the distal stomach is best achieved by starting on the greater curvature. The gastrocolic ligament is identified and incised to enter the lesser sac (FIG 3). Downward traction on the transverse colon and upward traction on the stomach will help expose this plane (FIG 4). Identification of the right and left gastroepiploic vessels along the greater curvature is essential. In benign disease, the plane of dissection can be very close to the stomach inside the gastroepiploic vessels. A large part of this plane is avascular and can be divided with electrocautery, whereas encountered v essels should be divided between clamps and ligated with 3-0 silk Mulholland_Part2_Ch10.indd 4 n ligatures. Once the lesser sac is identified, electrosurgical devices can be used to further mobilize the greater curvature (FIG 5). Proximally, dissection is carried to the midpoint of the greater curvature preserving the left gastroepiploic artery. Distally, the plane is developed beyond the pylorus to the duodenum and, once identified, the right gastroepiploic artery should be clamped, ligated with 2-0 silk ligatures, and divided. Here, one should be aware of the underlying pancreatic tissue and dissection should be meticulous. Dissection along the greater curvature allows entrance to the lesser sac, and the stomach can be lifted superiorly exposing its posterior surface and the congenital 1/24/14 5:18 PM C h a p t e r 10 ANTRECTOMY Right gastric artery Left gastroepiploic artery Liver Stomach Right gastroepiploic artery A Plane of dissection Pancreas er L s es c sa TECHNIQUES Left gastric artery 5 Colon B FIG 3 l Dissection of the greater curvature. A. Division of the greater omentum along the greater curvature will allow access to the lesser sac. The gastroepiploic vessels should be identified and care should be taken to not damage the transverse colon and its mesentery. B. Cross-sectional view of the upper abdomen demonstrating the plane of dissection that allows entrance to the lesser sac and mobilization of the greater curvature. ttachments to the underlying pancreatic capsule a (FIG 6). These attachments should be sharply divided. As this plane is developed in a superior direction, great care should be taken to not injure the left gastric artery at its origin from the celiac axis. Inflammation and scarring can be encountered in the setting of posterior gastric wall ulcers. Attention is then turned to division of the gastrohepatic ligament along the lesser curvature. Retracting the stomach inferiorly and to the patient’s left facilitates exposure of the lesser curvature. This dissection can start in the t ransparent pars flaccida and is carried proximally to the incisura and distally to the right gastric artery, which should be clamped, ligated with 2-0 silk ligatures, and divided (FIG 7). Again, electrocautery or electrosurgical devices can be used along the lesser curvature. One must be aware of an aberrant or replaced left hepatic artery originating from the left gastric artery and traversing the gastrohepatic ligament. If encountered, attempts should be made to preserve this vessel. After clamping but before division of the right gastric artery, blood flow to the liver should be confirmed by palpation of the hepatoduodenal ligament. FIG 4 l Traction on the stomach and the colon will aid dissection through the gastrocolic ligament allowing entrance to the lesser sac. As seen here, the lesser sac will be a true space that lies deep to the greater omentum. FIG 5 l Once the lesser sac is identified, the greater curvature can be dissected both proximally and distally taking care to identify the gastroepiploic vessels. n Mulholland_Part2_Ch10.indd 5 1/24/14 5:18 PM 6 TECHNIQUES P a r t 2 OPERATIVE TECHNIQUES IN GASTROINTESTINAL SURGERY FIG 7 l Dissection of the lesser curvature. FIG 6 l Dissection of the greater curvature allows for superior retraction of the stomach exposing its posterior surface and the underlying pancreas. Here, the lesser curvature and the left gastric artery are seen from the posterior aspect of the stomach. DIVISION OF THE STOMACH n n A stapling device is used to divide the stomach along a plane from just proximal to the incisura angularis on the lesser curvature to a point on the greater curvature twothirds of the way from the gastroesophageal junction to the pylorus. Some recommend placing Babcock forceps distal to the staple line to prevent sliding or rotation of the gastric mucosa as the stapler is closed. This will help ensure a clean, even cut across the anterior and posterior layers of gastric mucosa (FIG 8). Division of the duodenum varies based on the type of reconstruction planned. The duodenum is divided between Potts clamps for a Billroth I procedure in order to facilitate the gastroduodenal anastomosis. If possible, a stapling device should be used to divide the duodenum in a Billroth II procedure. Duodenal ulcer FIG 8 l Division of the stomach. RECONSTRUCTION: GASTRODUODENOSTOMY (BILLROTH I) n n If a Billroth I anastomosis is planned, a Kocher maneuver is needed to mobilize the duodenum. The duodenum is transected distal to the diseased area but proximal to the ampulla between Potts clamps, and the specimen is handed off the sterile field (FIG 9). It is essential that all gastric antrum be resected to prevent Mulholland_Part2_Ch10.indd 6 n persistent ulcer disease from retained gastric antrum. If there is question, a frozen section can be sent to confirm duodenal tissue at the resection line. At this point, one can gauge the mobility of the stomach, and if limited, the attachments of the fundus to the base of the diaphragm can be divided along with the gastrosplenic ligament taking care to preserve the left gastroepiploic vessels. This further mobilization should allow for a tension-free gastroduodenal anastomosis. If there 1/24/14 5:18 PM C h a p t e r 10 ANTRECTOMY FIG 9 l Division of proximal duodenum. A B TECHNIQUES n is concern about the ability to perform a tension-free anastomosis due to difficulty mobilizing the duodenum or the stomach, then a Billroth II procedure should be performed. For a Billroth I procedure, the duodenal stump is cleared of adjacent adipose tissue for roughly 1.5-cm distance in preparation for an end-to-end anastomosis. This anastomosis is constructed in standard two-layer fashion. Silk 3-0 seromuscular “stay” sutures are placed at the ends of the anastomosis to approximate the duodenum to the inferior aspect of the gastric staple line and the posterior layer is completed with interrupted 3-0 silk seromuscular sutures. Next, the inferior portion of the gastric staple line is removed using electrocautery for a length that correlates with the width of the duodenal stump. Two running 3-0 polydioxanone (PDS) sutures are used for the inner layer and an anterior layer of interrupted 3-0 silk seromuscular sutures completes the anastomosis. The remaining gastric staple line can be oversewed with interrupted 3-0 silk sutures (FIG 10). 7 C FIG 10 l A–C. Billroth I. RECONSTRUCTION: GASTROJEJUNOSTOMY (BILLROTH II) n n In performing a gastrojejunostomy, the gastric staple line is oversewed with interrupted 3-0 silk sutures. Next, the duodenum is divided distal to any disease with a stapling device. If it is not possible to fit a stapler into this plane, the duodenum can be divided with electrocautery and closed with a running 3-0 PDS. To buttress this closure, 3-0 silk full-thickness sutures can be placed and left untied. Omentum can be mobilized and loosely secured in place over the closure with the silk suture ends. Mulholland_Part2_Ch10.indd 7 n n We preferentially perform an isoperistaltic, retrocolic gastrojejunostomy, although an antecolic anastomosis is widely accepted. In the setting of malignancy, an antecolic approach may be favored as concern exists that progression of disease and future diffuse mesenteric lymphadenopathy may obstruct a retrocolic anastomosis. The anastomosis should be performed as close to the ligament of Treitz as possible (usually 10 to 15 cm), allowing for a tension-free anastomosis to minimize the risk of developing afferent limb syndrome. Although we prefer a stapled anastomosis, both stapled and hand-sewn techniques are widely accepted with similar rate of postoperative complications. A 45-mm stapled 1/24/14 5:18 PM 8 TECHNIQUES P a r t 2 OPERATIVE TECHNIQUES IN GASTROINTESTINAL SURGERY A B FIG 11 l A,B. Billroth II. gastrojejunal anastomosis is formed by creating a posterior wall gastrotomy and an antimesenteric enterotomy. Care is taken to ensure that the anastomosis is away from the prior gastric staple line on the posterior wall of the stomach. The common enterotomy is then closed with a 3-0 PDS full-thickness layer and an overlying layer of interrupted 3-0 silk sutures (FIG 11). Interrupted 3-0 silk sutures are then used to close the colonic mesentery defect to prevent bowel herniation (FIG 12). FIG 12 l Retrocolic gastrojejunostomy. PEARLS AND PITFALLS Indication Today, there is no role for antrectomy in treatment of noncomplicated peptic ulcer disease. A vast majority of peptic ulcers complicated by hemorrhage or perforation can be controlled with less invasive procedures. n n Operative planning n Laparotomy n Gastric mobilization n Duodenal transection n Mulholland_Part2_Ch10.indd 8 Preoperative endoscopy is essential to evaluate for the scope of disease and to allow biopsy of persistent gastric ulcers to rule out malignancy. n In the setting of malignancy, preoperative staging is warranted and a more extensive, oncologic resection may be indicated. Prior to extensive dissection, the extent of disease should be gauged to determine the operative plan and feasibility of a Billroth I anastomosis. With mobilization of the greater curvature, the middle colic vein is at risk of injury. Caudal traction on the transverse colon during dissection can help prevent an inadvertent injury. Great care should be taken to not fracture or injure the head of the pancreas, which can be closely adherent due to chronic inflammation. n If the common bile duct is difficult to identify, a cholecystectomy can be performed and a catheter can be placed in the cystic duct. Palpation of the catheter can aid in safely identifying the portal structures during dissection of the duodenum. 1/24/14 5:18 PM 9 C h a p t e r 10 ANTRECTOMY Reconstruction Billroth I and II procedures are performed with acceptable postoperative morbidity. A Billroth II anastomosis should be performed if there is concern about mobility of the duodenum or the stomach. n A short afferent limb (10 to 15 cm from the ligament of Treitz) can help minimize the likelihood of significant postoperative complications. n n POSTOPERATIVE CARE Unless concerning comorbidities exist, patients can be monitored on the hospital floor/ward. A nasogastric tube is positioned intraoperatively in the proximal stomach and typically can be removed on postoperative day (POD) 1. There is no role for postoperative antibiotic prophylaxis. Unless contraindicated, all patients receive chemical prophylaxis for deep vein thrombosis and are encouraged to ambulate on POD 1 and begin pulmonary toilet with incentive spirometry. There is no convincing evidence for routinely placing abdominal drains after Billroth I and Billroth II procedures. If there is concern for the GI anastomosis or adequate closure of the duodenal stump, a closed suction abdominal drain can be placed. Oral intake is reintroduced on POD 3 and advanced as tolerated. n Dumping—Early dumping presents as crampy abdominal pain and diarrhea shortly after eating due to the large hyperosmolar load of simple sugars which quickly enter the small bowel in the absence of a pylorus. Late dumping occurs roughly 2 hours postprandial with the symptoms of hypoglycemia likely due to insulin response to the large sugar bolus. Dumping occurs in 5% of postgastrectomy and is controlled with diet modifications, and rarely, octreotide is given with success in severe and refractory cases. n Retained gastric antrum—Incomplete antrectomy with retained G cells within the duodenal stump can result in recurrent ulceration from continued intense gastrin secretion. Exposure of the jejunum to high levels of acid results in an anastomotic or marginal ulcer. A sodium 99m technetium scan identifies antral tissue and reexcision is needed for complete symptom relief. n OUTCOMES REFERENCES Serious morbidity from postgastrectomy syndromes develops in 3% to 5% of patients. n Thirty-day mortality for uncomplicated gastric ulcer disease is 1% to 2% and increases in emergency settings.11 1. Glasgow RE, Rollins MD. Stomach and duodenum. In: Norton JA, Barie PS, Bollinger RR, et al, eds. Surgery: Basic Science and Clinical Evidence. 2nd ed. New York, NY: Springer; 2008:841–874. 2. Gray RJ, Kelly KA. Peptic ulcer. In: Kelly KA, Sarr M, Hinder R, eds. Mayo Clinic Gastrointestinal Surgery. Philadelphia, PA: Saunders; 2003:103–124. 3. Zittel TT, Jehle EC, Becker H. Surgical management of peptic ulcer disease today—indication, technique, and outcome. Langenbecks Arch Surg. 2000;385:84–96. 4. Bardhan KD, Royston C. Time, change, and peptic ulcer disease in Rotherdam, UK. Dig Liver Dis. 2008;40(7):540–546. 5. Lundell L. Acid secretion and gastric surgery. Dig Dis. 2011;29(5): 487–490. 6. Lipof T, Shapiro D, Kozol RA. Surgical perspectives in peptic ulcer disease and gastritis. World J Gastroenterol. 2006;12(20):3248–3252. 7. Doherty GM, Way LW. Stomach and duodenum. In: Doherty GM, ed. Current Diagnosis & Treatment: Surgery. 13th ed. New York, NY: McGraw-Hill; 2010. 8. Mulholland MW. Gastroduodenal ulceration. In: Mulholland MW, Lillemoe KD, Doherty GM, et al, eds. Greenfield’s Surgery: Scientific Principles and Practice. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010. 9. Hou W, Schubert ML. Treatment of gastric carcinoids. Curr Treat Options Gastroenterol. 2007;10(2):123–133. 10. Chassin JL, Henselman C. Gastrectomy (antrectomy) for peptic ulcer. In: Chassin JL, Henselman C, eds. Chassin’s Operative Strategy in General Surgery: An Expositive Atlas. New York, NY: Springer- Verlag; 1994. 11. Siewert JR, Bumm R. Distal gastrectomy with Billroth I, Billroth II, or Roux-Y reconstruction. In: Fischer JE, Bland KI, eds. Mastery of Surgery. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:849–859. 12. Bolton JS, Conway WC II. Postgastrectomy syndromes. Surg Clin N Am. 2011;91:1105–1122. n COMPLICATIONS After an antrectomy, short-term complications include delayed gastric emptying, anastomotic leak, bleeding, and pancreatitis. Long-term complications include the postgastrectomy syndromes (described below) and anastomotic stricture. Additionally, chronic anemia, neuropathy, and osteopenia can result from iron, copper, and calcium malabsorption due to bypassing of the proximal small bowel in a Billroth II procedure.12 n Postgastrectomy syndromes n Afferent loop syndrome n Postprandial right upper abdominal colicky pain that accumulates in bilious emesis that alleviates the pain n Results from chronic dilation, obstruction, or stasis of the duodenum (afferent limb) after a Billroth II procedure n Rarely occurs but can be corrected by revision of the Billroth II, conversion to a Roux-en-Y reconstruction, or performing an afferent to efferent bypass (Braun enteroenterostomy) n Reflux gastritis—Patients report epigastric burning pain resulting from reflux of bile into the stomach. As expected, bile reflux is more common after Billroth I and Billroth II procedures than Roux-en-Y reconstruction and if severe can be treated by conversion to a Roux-en-Y reconstruction. n Mulholland_Part2_Ch10.indd 9 1/24/14 5:18 PM