* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Quantum-Mechanical Model of the Atom
Hartree–Fock method wikipedia , lookup
Wheeler's delayed choice experiment wikipedia , lookup
Copenhagen interpretation wikipedia , lookup
Probability amplitude wikipedia , lookup
Quantum chromodynamics wikipedia , lookup
Chemical bond wikipedia , lookup
Delayed choice quantum eraser wikipedia , lookup
Hidden variable theory wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Molecular orbital wikipedia , lookup
Hydrogen atom wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Elementary particle wikipedia , lookup
Double-slit experiment wikipedia , lookup
Tight binding wikipedia , lookup
Atomic orbital wikipedia , lookup
Matter wave wikipedia , lookup
Electron configuration wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
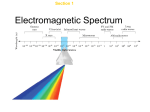
8/27/12 The Quantum-‐Mechanical Model of the Atom We shall be using the Atoms-‐First Approach! We shall first start with the pHundamentals! • Atoms are the fundamental parDcles of chemistry. • Atoms are composed of proton, neutrons, and electrons. – Electrons are where the “acDon” is in chemistry. – But the other parDcles are important, too! • Some of these parDcles, in turn, are composed of others. Now, before we go further... • It is my job to – explain what is in the book AND – to supplement your knowledge! • Thus, I want none of... 1 8/27/12 The Theory we Shall Dabble in is Quantum Mechanics • This is the theory of the “very small.” • What do we mean by “small”? – The smallest measurement that has physical meaning is called the “Planck length” and is about 1.616199 × 10-‐35 meters. – An atom is about 10-‐10 meters in diameter. • So, what would “large” be? – The universe is about 10+28 meters wide. ParDcles within parDcles... • Atoms are made of protons, neutrons, and electrons. • Electrons are fundamental in their own right and are one type of lepton. • But protons and neutrons are made up of even smaller parDcles called quarks. Quarks & Leptons There are 6 basic quarks. There are 6 basic leptons. Each of these has an anDparDcle. Thus, there are 24 fundamental parDcles (for mass) in nature. • The next slide gives a list of all of them. • • • • 2 8/27/12 The List! What holds these together? • There is a set of force-‐carrying parDcles, too! • These are listed according to the various forces in nature: – Gravity (gravitons) – Weak force (W & Z bosons, the only fundamental force parDcles with mass) – ElectromagneDsm (photons) – Strong force (gluons) The photon as a force carrier... 3 8/27/12 For pHun, here is a weak force carrier in acDon... What goes on can be very complex (we won’t discuss this one!) Here is a summary for atomic parDcles... 4 8/27/12 Ordinary maher is made of... • • • • • Protons, Neutrons, Electrons, and the relevant force carriers. All other parDcles serve no purpose that anyone knows of... There is even a song about this! • Put in ear plugs if you can’t stand this... • Also shut your eyes if you are too young to see this... hhp://www.youtube.com/watch? v=D3wBV1KN6FQ Of course, one could get even more intellectual about this... • One guy decided that a rap was needed. • So... hhp://www.youtube.com/watch?v=xYZkj2FPeoc 5 8/27/12 Here are the innards of a proton... (Note the colors!) Protons and neutrons... • Belong to a class of parDcles called baryons. • All baryons are composed of three quarks. – Protons are made to two up quarks and one down quark. – Neutrons are made of one up quark to two down quarks. pHinally, the Nuclear Force! • Gluons hold quarks together to make protons and neutrons. • Protons and neutrons, in turn are held together by the nuclear force, which is derived from the strong force. • Protons and neutrons are held together by pions; each pion composed of a quark and an anD-‐quark! 6 8/27/12 Here are a proton and neutron being ahracted... This is the last parDcle type we need to know about! • Let’s here it for quarks, leptons, and all those happy force-‐carrying bosons! hhp://www.youtube.com/watch? v=Ez9f5EQ84Tg Here is a summary of what is in the atom... 7 8/27/12 Of course, it is wise to read the pHine print! Some History! • Quantum mechanics began as a basically a classical descripDon of problems with black body radiaDon. • Black body radiaDon explains the radiaDon given off by warm objects (for instance, all red hot metals are at about the same temperature). • But there was a fundamental problem! The Ultraviolet CATastrophe! 8 8/27/12 What is that? • According to classical physics, every object with T > 0 K should radiate an infinite amount of energy. • This was very disturbing! Here is what a BB curve is... BB radiaDon explains the colors of hot objects! And, below is what actually happens! 9 8/27/12 The problem was solved by Max Planck in 1900... What Planck did... • He found an equaDon that fit the data. • This equaDon forced vibraDonal frequencies to be mulDples of a fundamental frequency instead of conDnuous. Energy is quan-zed! • He put in an adjustable parameter, h. • This parameter was later on found to be a fundamental physical constant! Let’s look at the nature of light... • Light is a type of electromagne7c radia7on. • All radiaDon of this type is composed of oscillaDng electric and magneDc fields perpendicular to each other. 10 8/27/12 Some Details • EM radiaDon in spectroscopy has simple sinusoidal waves. • Waves are characterized by amplitude and wavelength (λ). • We see an example to the right. Some relaDonships... • Light has a speed of propagaDon, this is c = 299792458 m/s. • This is an exact number! • The wavelength in turn —along with c—allows us to define the frequency (ν) of the wave. The Range of Frequencies Leads to the Electromagne7c Spectrum 11 8/27/12 Each type of radiaDon corresponds to a type of atomic/molecular interacDon (Hence the importance of spectroscopy in chemistry!) Radia%on Type Atomic/Molecular Ac%on Radio waves Nuclear MagneDc Resonance Microwave Molecular RotaDon Infrared Molecular VibraDons Visible/Ultraviolet Outer electron excitaDons X-‐ray Inner electron excitaDons γ-‐ray Nuclear excitaDons Two important aspects of the wave nature of EM radiaDon... • Interference (construcDve and destrucDve) • DiffracDon Look at interference first... Construc%ve Interference Destruc%ve Interference 12 8/27/12 DiffracDon occurs because of the wave nature of light... DiffracDon and interference together are more than twice the pHun! The parDcle nature of light... Isaac Newton thought that light was a parDcle. He called these parDcles, “corpuscles.” 13 8/27/12 However... • The wave model of Thomas Young proved to be much more powerful. • It explained diffracDon (which Newton knew nothing about). • Young looks very pleased about this! The truth is more complicated! • Light acts both as a wave and as a parDcle! • It is composed of massless parDcles called photons. • This came from the photoelectric theory of Einstein. • He put Newton and Young in their places! This was a revoluDon! • Young’s theory was refined extensively by James Clerk Maxwell. • This seemed to solve all problems dealing with radiaDon. • Some people even thought that physics was done. • Maxwell probably doubted this. 14 8/27/12 But we sDll admire his equaDons! What is the photoelectric effect? What was wrong... • Maxwell’s theory said that the energy of electrons coming off the metal surface should be related to the amplitude of the wave. • However, this was not true. • Einstein found a different—and very simple— relaDonship. • We look at examples on the next slide. 15 8/27/12 Two examples... Results for three metals (note the iden%cal slopes!) Data for a single metal And the slope was... h In general, the kineDc energy of the ejected electrons is... 16 8/27/12 Photons • Light comes in the form of photons. • Photons are massless parDcles. • Photons have definite energy and momentum! • Photons are both a parDcle and a wave! And a lihle more to say... • Remember that we consider both frequency and wavelength. • So, let’s combine our rules and come up with a more useful set of equaDons. h is a fundamental physical constant! 17 8/27/12 What exactly, then, is a photon? • It is a parDcle. • It is a wave. • SomeDmes, it is drawn as shown to the right... The depicDon below may be more “realisDc”! Some points about wave-‐parDcle duality... • Is this really a strange concept? • Why not just accept it as the way things are? • In some cultures such duality may not be strange at all; just look to the right! 18 8/27/12 If you can’t understand, laugh! We have seen h appear in two places! • Black body radiaDon • The photoelectric effect • This gives us some suspicion that h is universal. • The next slide shows yet another appearance of this now seemingly ubiquitous constant. Atomic Spectra led to the next appearance of h... • Every element has a unique atomic spectrum. • These are most easily observed via gas discharges. • Some examples are to the right. 19 8/27/12 A prism or graDng can split the light from an element into a SPECTRUM Why is this happening? The answer was found by Niels Bohr. The old bore is shown to the right... What Bohr showed... • Electrons in an H-‐atom are confined to specific orbitals. • Planck’s constant figures into the orbital energies. • Only discrete frequencies can be absorbed or emi=ed. 20 8/27/12 More detail... • Bohr got this (h and other constants are embedded in R). • m and n are integers. R = 10973731.568525m-‐1 (R is ozen labeled as RH for reasons that will be obvious later.) Bohr’s Complete EquaDon for R Various values for R... 21 8/27/12 Wow! Look at where h is... • • • • • • Black body radiaDon. The photoelectric effect. The definiDon of the photon and its energy. Rydberg’s constant. Is there more? Mais oui, mes amis! Maher as a wave! • If light is both parDcles and waves and • quantum mechanics is the theory of the very small, • then, maybe small parDcles act as waves. • This was shown by Prince Louis de Broglie! de Broglie put some things together in a strange way... For maBer For photons 22 8/27/12 Now, do some devious combining! Bad math-‐-‐but good science! • de Broglie’s equaDon came strictly from analogy. • But it is true! • This was shown to be so by Davisson & Germer who first demonstrated electron diffracDon. Now, since parDcles have wavelike moDon... • How can we say exactly where a parDcle is? • That is, look at the picture at the right; where, exactly, is the parDcle? 23 8/27/12 The Uncertainty Principle • This was first proposed by Werner Heisenberg. • The old buzzard is to the right. • (Actually, he was a spring chicken when this picture was taken.) Basic Idea of the U.P. • The is an inherent limit in how well we can know the values of two complementary physical properDes. • Complementary properDes have combined units of acDon (energy × Dme). ImplicaDons... • The higher the precision of one observable, the more uncertain the other. • This tradeoff is shown to the right! 24 8/27/12 Spectrum line widths... • In passing, we also note implicaDons about frequency and Dme. • We shall look at a short discussion of this to the right. • Note that width at half-‐ height of a spectrum line is wrihen as δν. The main implicaDons... • In many cases, we cannot know a quanDty exactly. • Rather, we have to live with staDsDcs! • The wave nature of maher thus dominates things at the atomic/molecular level! • We shall see that, in fact, we can visualize things as standing waves. Maybe this says it all... 25 8/27/12 The wave model turned out to be the key to understanding atoms... • The Bohr model was limited. • What was needed was a more general model. • This came about from the work of Erwin Schrödinger. • The next slide is the Schrödinger equaDon for the hydrogen atom. The whole thing... Here is Schrödinger and a simplified form of the equaDon... 26 8/27/12 How this equaDon solved many problems... • The wave characterisDcs of maher were clearly included and defined. • The energies were correctly determined; these were the same as the Bohr model but on a much firmer fooDng. • Other things such as the angular momentum of the electron orbits naturally emerged from the soluDon. All this devolved into 3 simple quantum numbers! • n: The principal quantum number • l: The azimuthal quantum number • ml: The magneDc quantum number. n give the energy of the H-‐atom 27 8/27/12 This is easy to graph... l defines the shape of the orbital • l is determined by n. • 0 ≤ l ≤ n – 1 Ø n = 1 → l = 0 Ø n = 2 → l = 0, 1 Ø n = 3 → l = 0, 1, 2 Some special nomenclature... • • • • l = 0: s orbitals (“sharp”) l = 1: p orbitals (“principle”) l = 2: d orbitals (“diffuse”) l = 3: f orbitals (“fine”) 28 8/27/12 ml defines the direcDon of an orbital... • • • • • • ml is defined by l. -‐l ≤ ml ≤ +l l = 0: ml = 0 l = 1: ml = -‐1, 0, +1 l = 2: ml = -‐2, -‐1, 0, +1, +2 ml takes on 2l + 1 values for a given l. Atomic Spectroscopy Explained! • n prehy much fills the bill here (at least for one-‐electron atoms). • Looking to the right, you see absorp7on and emission. Here is a more detailed look... 29 8/27/12 Orbitals and Wave FuncDons • ψ2 corresponds to a probability density. • We very ozen think of electron density in terms of this probability density. • There are various ways to envision this. Probability DepicDons (1s) Dot Densi%es Probability Density Surface DescripDons (1s) Orbital Surface (90%) Radial Distribu%on Func%on 30 8/27/12 More complicated orbitals ozen have nodes. (We see these first in a string.) For pHun, there is an alternate way to express this... Nodes in the 2s and 3s Orbitals 31 8/27/12 Things get more interesDng when we get to p orbitals... d orbitals are even more pHun! f orbitals re really out there! 32 8/27/12 In math, these are the first few spherical harmonics! pHinal comments on interference and waves... • SomeDmes, when we have construcDve interference, we say that waves are “in phase.” • Otherwise, we might say that they are “out of phase.” • The next slide shows a simple example of this. Simple example of phase... 33 8/27/12 This applies to 3D waves, too (Note the use of color) Quick comment... Do the ideas of construcDve and destrucDve interference and the idea of phase give you— just maybe—and inkling of why atoms might want to bond together? 34