* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Congenital hyperinsulinism
Survey
Document related concepts
Transcript
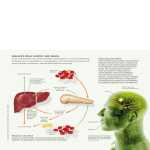
Seminars in Fetal & Neonatal Medicine (2005) 10, 369e376 www.elsevierhealth.com/journals/siny Congenital hyperinsulinism K. Hussain* The Institute of Child Health, Unit of Biochemistry, Endocrinology and Metabolism, University College London, 30 Guilford Street, London WC1N 1EH, UK KEYWORDS Congenital hyperinsulinism; Hypoglycaemia; Insulin; Glucose; Potassium channels; SUR1; KIR6.2 Summary Congenital hyperinsulinism is a cause of persistent hypoglycaemia in the neonatal period. It is a heterogeneous disease with respect to clinical presentation, molecular biology, genetic aetiology and response to medical therapy. The clinical heterogeneity may range from severe life-threatening disease to very mild clinical symptoms. Recent advances have begun to clarify the molecular pathophysiology of this disease, but despite these advances treatment options remain difficult and there are many long-term complications. So far mutations in five different genes have been identified in patients with congenital hyperinsulinism. Most cases are caused by mutations in genes coding for either of the two subunits of the b-cell KATP channel (ABCC8 and KCNJ11). Two histological subtypes of the disease e diffuse and focal e have been described. The preoperative histological differentiation of these two subtypes is now mandatory as surgical management will be radically different. The ability to distinguish diffuse from focal lesions has profound implications for therapeutic approaches, prognosis and genetic counselling. ª 2005 Elsevier Ltd. All rights reserved. Introduction Congenital hyperinsulinism (CHI) is the most common cause of persistent and recurrent hypoglycaemia in the neonatal period. For decades, the disease was thought to be due to ‘nesidioblastosis’. This term, which was first used by Laidlaw, describes the persistence of a diffuse * Tel.: C44 20 7 905 2128; fax: C44 20 7 404 6191. E-mail address: [email protected] and disseminated proliferation of islet cells budding off from the pancreatic ductal tissue.1 However, it is now quite clear that nesidioblastosis is a common feature of the pancreas in normoglycaemic neonates.2 Neonatal onset CHI is a major risk factor for development of severe mental retardation and epilepsy.3 Biochemically, CHI is characterized by inappropriate and unregulated insulin secretion from pancreatic b-cells. The biochemical profile at the time of hypoglycaemia reflects the metabolic actions of insulin. The dysregulated and uncontrolled release 1744-165X/$ - see front matter ª 2005 Elsevier Ltd. All rights reserved. doi:10.1016/j.siny.2005.03.001 370 of insulin from the b-cells reflects the final manifestation of a number of different processes that alter intracellular biochemical pathways of the bcell, thereby generating abnormal signals for the secretion of insulin.4 These abnormalities perturb the normal physiological mechanisms which normally ensure that the amount of insulin secreted is directly related to the ambient blood glucose concentration. CHI is characterized by the presence of insulin and c-peptide concentrations that are inappropriately high for the level of blood glucose. A ‘normal’ insulin level for normoglycaemia is usually inappropriate in the presence of hypoglycaemia, especially taken in the context of high glucose requirement to maintain normoglycaemia.5 There appears to be very little correlation between circulating levels of insulin at presentation and the severity of hypoglycaemia.5 Recent advances have begun to unravel the pathophysiology of this intriguing disease as well as providing an understanding of the normal physiological and biochemical mechanisms regulating insulin secretion from pancreatic b-cells. It is now clear that CHI is a heterogeneous disorder with respect to clinical presentation, histology, molecular biology and genetics.6 The histological differentiation of focal and diffuse forms of CHI has radically changed the surgical approach to this disease, and the focal form of the disease can now be cured by partial pancreatectomy.7,8 So far, mutations in five different genes have been described which lead to dysregulated insulin secretion from b-cells.9e13 Despite these advances the genetic defect is still unknown in about 50% of cases. Clinical presentation and diagnosis Typically, CHI presents in the neonatal period, usually within the first few days of birth, although it can present later in infancy and childhood.14 Neonates will present with specific symptoms (such as fits) or non-specific symptoms (lethargy, irritability, poor feeding) of hypoglycaemia. Macrosomia may be a feature in some patients, but not all neonates with CHI are macrosomic. The hypoglycaemia in CHI is persistent and recurrent, and in most cases normoglycaemia can only be maintained by giving large volumes of intravenous glucose. CHI can occur in preterm babies, in which case it appears to be more severe and aggressive.15 Hypertrophic cardiomyopathy is a common clinical finding in patients with CHI, but the underlying mechanism for this is unclear. K. Hussain Hyperinsulinism can also be transient. The transient form is associated with infants born to mothers with diabetes mellitus (gestational and insulin-dependent), in infants with Beckwithe Weidemann syndrome, in intrauterine growth retardation babies, and in babies subjected to perinatal asphyxia.16 Transient hyperinsulinism may also occur in babies where there is no predisposing factor.17 The mechanisms causing transient hyperinsulinism in these conditions are not clear. Hyperinsulinism may also be a manifestation of a rare syndrome such as Kabuki, Costello or Turner’s syndrome (Hussain K, unpublished observations) or congenital disorders of glycosylation18 and some undiagnosed syndromes.19 Foregut dysmotility and gastro-oesophageal reflux is common in CHI.20 The pathophysiology of the gut dysmotility and the gastro-oesophageal reflux is unclear. Infants may show poor sucking and swallowing, retching, vomiting, and intestinal dilatation; feeding problems are compounded by the use of nasogastric or gastrostomy feeds, which delay the establishment of normal feeding patterns, taste, and ‘orality’. In severe cases, the deprivation of oral stimulation may require months of rehabilitation with skilled speech and language therapists. The biochemical hallmark of CHI is hyperinsulinaemic, hypoketotic, hypofattyacidaemic hypoglycaemia. These biochemical abnormalities reflect the metabolic actions of insulin. The unregulated insulin secretion causes increased glucose disposal in insulin-sensitive tissues such as the liver (hepatomegaly on clinical examination), adipose tissue and skeletal muscle, and simultaneously inhibits glucose production. There is also higher glucose demand in the absence of alternative fuels. This is reflected in the increased glucose clearance rate (above the normal of 5 mg/kg/min) required to maintain normoglycaemia. The serum cortisol counter-regulatory hormone levels are blunted in hyperinsulinaemic hypoglycaemia due to the lack of drive from the hypothalamicepituitary axis, and replacement therapy with glucocorticoids does not seem to affect the severity of the disease.21 In most cases the diagnosis of CHI is relatively straightforward, but in difficult cases other supportive evidence is required: for example, decreased serum levels of insulin growth factor binding protein 1 (IGFBP-1) (as insulin suppresses the transcription of the IGFBP-1 gene),22 a positive glycaemic response to intramuscular/intravenous glucagon at the time of hypoglycaemia (a clear increment in blood glucose concentration despite severe hypoglycaemia),23 and a positive glycaemic Congenital hyperinsulinism response to octreotide with a concomitant reduction in the glucose infusion rate required to maintain normoglycaemia. 371 KIR 6.2 (KCNJ11gene) SUR 1 (ABCC8 gene) ATP/ADP Pathophysiology of CHI Under normal physiological conditions the pancreatic b-cells are exquisitely sensitive to the plasma glucose concentration and secrete appropriate amounts of insulin. Pancreatic b-cells possess a signal transduction system whereby metabolic changes in the b-cell are linked to regulated insulin secretion.24 The metabolic changes are linked to regulated insulin secretion by potassium channels (KATP) located in the pancreatic b-cell membrane.25 Each KATP channel consists of a heteromultimeric complex of at least two proteins designated SUR1 (ABCC8 gene) and KIR6.2 (KCNJ11 gene).26 The functional integrity of both of these proteins is necessary for potassium channel movement, and the genes responsible for them have been localized very closely to each other on the short arm of chromosome 11 (11p14-15.1). Under normal physiological conditions the KATP channels maintain the electrical potential of the b-cell membrane. The metabolism of glucose in the b-cell increases the ratio of ATP/ADP, which has the effect of closing the KATP channels. This in turn causes the opening up of voltage-gated calcium channels, which regulate the entry of calcium into the bcell. The entry of calcium is thought to be the final stimulus for insulin exocytosis.25 Fig. 1 illustrates the role of KATP channels and voltage-gated calcium channels in regulating insulin secretion. Although KATP channels have an essential role in linking the metabolism of glucose to the secretion of insulin, there is now evidence that there may well be other mechanisms of insulin secretion: the so-called KATP channel-independent pathways of insulin secretion.27 The commonest genetic cause of persistent CHI is an abnormality of the KATP channels.28 A number of mutations in the ABCC8 and KCNJ11 genes have been defined, particularly in children with the familial forms of CHI.9,10 These mutations impair the function of the KATP channel by affecting channel density, channel expression, channel trafficking from the Golgi apparatus and endoplasmic reticulum, channel gating properties and channel activity in response to changes in the concentrations of intracellular nucleotides. Rare causes of CHI include gain-of-function mutations (autosomal dominant) in the genes encoding the enzymes glucokinase (GK) and glutamate GLUCOSE Ca2+ Ca2+ Mitochondria Pancreatic β-cell EXOCYTOSIS Figure 1 KATP channels link glucose metabolism to regulated insulin secretion. The KATP channel responds to changes in the concentration of intracellular nucleotides such as ATP/ADP. Glucose metabolism in the pancreatic b-cell increases the ATP/ADP ratio. This has the effect of closing the KATP channels, allowing the entry of calcium. The entry of calcium is the trigger for exocytosis of insulin. The commonest causes of CHI are mutations in the genes (ABCC1 and KCNJ11) that regulate the function of the two proteins of the KATP channels (SUR1 and KIR6.2, respectively). dehydrogenase (GDH).11,12 CHI due to abnormalities in these enzymes is usually less severe and often presents late. GK is a key glycolytic enzyme that functions as a glucose sensor in the pancreatic b-cell, where it governs glucose-stimulated insulin secretion. GK mutations alter the threshold of glucose-stimulated insulin release and glucose homeostasis. The gainof-function mutation in glucokinase causes increased affinity of glucokinase for glucose, with increased rates of glycolysis at low blood glucose concentrations. This then increases insulin secretion at any given blood glucose concentration. GDH is a mitochondrial enzyme that catalyses the oxidative deamination of glutamate to aketoglutarate plus ammonia, using NAD or NADP as cofactor. The a-ketoglutarate then enters the Kreb’s cycle to produce ATP. The syndrome of hyperinsulinism/hyperammonaemia (HI/HA) is associated with dominantly expressed missense mutations in GDH. HI/HA mutations impair GDH sensitivity to its allosteric inhibitor GTP, resulting in a gain of enzyme function and increased sensitivity to its allosteric activator leucine. The clinical picture is characterized by postprandial hypoglycaemia following protein meals, as well as fasting hypoglycaemia. Plasma ammonia levels are increased to 3e5 times normal, but the mechanism of this is unclear. Another novel form of CHI recently described is due to autosomal recessive mutations in the gene encoding the enzyme short-chain L-3-hydroxyacylCoA dehydrogenase (SCHAD).13,29 This enzyme is 372 involved in the penultimate pathway of fatty acid b-oxidation. This defect provides the first clue to the in vivo dysregulation of insulin secretion by defects in the metabolism of fatty acids. The precise mechanism of dysregulated insulin secretion in children with SCHAD deficiency is not understood. Management Neonatal CHI is one of the most difficult diseases to manage in paediatric endocrinology. Patients should be referred to centres that have the necessary experience and expertise in managing this condition. These patients will require frequent and accurate blood glucose monitoring with prompt diagnosis and aggressive management of the hypoglycaemia. The goals of management include preventing hypoglycaemic brain damage, allowing normal psychomotor development, establishing a normal feeding pattern (content and frequency for the age of the child), ensuring normal tolerance to fasting for age without developing hypoglycaemia, and maintaining family integrity. Medical management The immediate imperative is to give sufficient glucose to maintain blood glucose concentrations above 3.5 mmol/L. Adequate carbohydrate can be provided as intravenous glucose in high concentrations, together with a nasogastric feeding tube for regular feeds. Glucose polymer can be added to the enteral feed to increase the carbohydrate intake but should be used with caution in neonates at risk of necrotizing enterocolitis. Some infants may require the insertion of a gastrostomy for regular and frequent feeds. Infusion rates in excess of 4e6 mg/kg/min are usually necessary. Rarely, infusion rates O20 mg/ kg/min may be needed.30 The delivery of concentrated glucose infusions will require the insertion of an umbilical venous catheter or central venous access. Having stabilized the blood glucose concentration, it is then imperative to determine whether the patient will respond to the conventional medical therapy. If there is no response to medical therapy the only other option is surgical. The mainstay of medical therapy is diazoxide. This drug works by locking onto the intact SUR component of the KATP channel. By keeping the channel open it stops insulin secretion. CHI due to abnormalities in the enzymes GK, GDH and SCHAD K. Hussain all respond to diazoxide, as these do not involve defects in KATP channels. However, patients with mutations in the KATP channel may not respond to diazoxide. The major side-effect of diazoxide in the short term is fluid retention. In the long term hirsutism is the main cosmetic side-effect. Chlorothiazide is used in conjunction with diazoxide for its hyperglycaemic action as well as to counteract the fluid-retaining properties of diazoxide.31 Given the role of the voltage-gated calcium channels in regulating insulin secretion, the use of a calcium-channel antagonist such as nifedipine has been proposed.32 However, despite several case reports documenting nifedipine-responsive CHI,33 the overall response to nifedipine has been disappointing. Glucagon and octreotide have several different roles in the management of an infant with CHI. Intramuscular glucagon (1 mg) can be used in the acute management of hypoglycaemia due to hyperinsulinism when intravenous access is unavailable. Glucagon acutely stimulates glycogenolysis, but also has actions on gluconeogenesis, lipolysis, protein degradation, amino acid catabolism and ketogenesis. Onset of action of glucagon is within 10e15 min. A subcutaneous or intravenous continuous infusion of glucagon (in combination with octreotide) is used in the short term to maintain stability until further investigations are planned. High doses of glucagon (O20 mg/kg/h) can cause paradoxical insulin secretion, which leads to worsening of the hypoglycaemia in patients with CHI.34 Octreotide is an analogue of somatostatin and is used in the short- and long-term management of patients with CHI. Somatostatin and its analogues inhibit insulin secretion by activation of somatostatin receptor 5, which is mediated by stimulation of the G-coupled proteins.35 Octreotide can be administered by subcutaneous injection or as a continuous subcutaneous infusion. Octreotide also causes the inhibition of the release of several hormones, including growth hormone (GH), serotonin, gastrin, vasoactive intestinal polypeptide (VIP), secretin, motilin, pancreatic polypeptide, ACTH, and thyroid-stimulating hormone (TSH). The suppression of GH (including insulin-like growth factors) and thyroid hormones may lead to stunting of growth, although in clinical practice this does not seem to be a problem. Octreotide can decrease gallbladder contractility and bile secretion leading to steatorrhoea, cholestasis, hepatic dysfunction and cholelithiasis. Blood flow to the splanchnic circulation is decreased by octreotide, hence it must be used cautiously in babies at risk of necrotizing entercolitis. Resistance to octreotide therapy can occur even at high doses. Congenital hyperinsulinism 373 The medications used in the treatment of CHI are summarized in Table 1. Surgical management Those children who fail to respond to medical therapy will require surgery. The extent of surgery will depend on the histological subtype of CHI. Two histological subtypes of CHI e diffuse and focal e have been described.2 The diffuse form is characterized by changes of b-cell hypertrophy and hyperplasia throughout the whole pancreas. The diffuse form is classically associated with defects in SUR1 and KIR6.2. The focal lesion is confined to one region of the pancreas and is distinct from an insulinoma.36 It is associated with a unique combination of events, with somatic loss of the maternal allele on the short arm of chromosome 11, in a child harbouring an SUR1 or a KIR6.2 mutation on the paternal allele.37,38 The juxtaposition of SUR1 and several imprinted genes on chromosome 11p15 appears to be responsible for this unique genetic mechanism of disease. About 40e50% of infants will have the focal form of the disease. It is now imperative to identify those children with the focal disease, as their management will Table 1 be radically different compared to those with diffuse disease. Those with focal disease will require a limited pancreatectomy with the aim of resecting only the focal lesion and preserving as much normal pancreatic tissue as possible. On the other hand those with diffuse disease will usually require a near-total pancreatectomy. Pancreatectomy is not without risk and is not a procedure to be undertaken lightly. Some children remain hypoglycaemic despite this, when a further attempt can be made to control the condition by diazoxide therapy. In a minority of cases, a total pancreatectomy may be necessary to control the severe hyperinsulinism. The risk of developing diabetes mellitus following a near-total pancreatectomy remains high.39 The typical diffuse form of the disease can now be identified by performing a laparoscopic tail biopsy of the pancreas (Hussain K et al., unpublished observation). Current methods of localizing focal lesions include intrahepatic pancreatic portal venous sampling40 and the intra-arterial calcium stimulation test.41 Even more recently 18F-fluoro-Ldopa PET (positron emission tomography) has been successfully used to localize the focal domain.42 This has many advantages over the highly invasive pancreatic venous sampling and intra-arterial calcium stimulation tests. Summary of the dietary, medical and surgical management of different forms of CHI Medication Route of administration Dose Mechanism of action Side-effects Diazoxide Oral 5e20 mg/kg/day divided into two or three doses Agonist of the KATP channel Fluid retention, hypertrichosis, hyperuricaemia, eosinophilia, leukopenia, rarely hypotension Chlorothiazide (used in conjunction with diazoxide) Oral 7e10 mg/kg/day divided into two doses Activation of KATP channels; synergistic response with diazoxide Hyponatraemia, hypokalaemia Nifedipine Oral 0.25e2.5 mg/kg/day divided into three doses Calcium channel blocker Hypotension Glucagon SC/IV infusion (Goctreotide) 1e20 mg/kg/h Increases glycogenolysis and gluconeogenesis Nausea, vomiting, paradoxical insulin secretion; skin rashes Octreotide SC/IV continuous infusion 6e8 hourly SC injections (Gglucagon) 5e25 mg/kg/day Multiple actions in the b-cell (see text) Suppression of GH, TSH, ACTH, glucagons; Diarrhoea, steatorrhoea, cholelithiasis, abdominal distension, growth suppression, tolerance GH, growth hormone; TSH, thyroid-stimulating hormone; ACTH, adrenocorticotrophic hormone. 374 Table 2 K. Hussain Summary of medical therapy used for the management of CHI Type of CHI Diet Medical Surgical Diffuse (KATP channel defect in SUR1 or KIR6.2) High calorie/frequent feeding D/C/N/G/O Near-total pancreatectomy Focal (KATP channel defect in paternal SUR1 or KIR6.2 with loss of heterozygosity) High calorie/frequent feeding D/C/N/G/O Limited pancreatectomy Glutamate dehydrogenase (GDH) up-regulation Protein restriction/high calorie D No surgery Glucokinase (GK) up-regulation High calorie D No surgery SCHAD deficiency High calorie D No surgery D, diazoxide; C, chlorothiazide; N, nifedipine; G, glucagon; O, octreotide. The medical and surgical treatment of CHI is summarized in Table 2. Mice knockout models of KATP channels and CHI Three mouse models have been developed to understand the role of KATP channels in the regulation of insulin secretion. These models include homozygous KIR6.2ÿ/ÿ knockout mice,43 the expression of a dominant negative mutant KIR6.2 subunit that reduces or eliminates KATP channel activity44 and SUR1 knockout.45 Transgenic mice are only mildly hypoglycaemic at birth, but become hyperglycaemic within 4 weeks as a result of b-cell destruction.44 The deletion of SUR1 or KIR6.2 from mouse pancreatic b-cells has unexpectedly little or no effect on glucose homeostasis, in contrast to the predicted hypoglycaemic phenotype seen in humans. The knockout animals do not have persistent hyperinsulinaemic hypoglycaemia despite the abolition of KATP channels. They may have a compensation mechanism using an as-yet-undefined KATP-independent regulatory pathway to maintain nearnormal insulin and blood glucose concentrations. The reasons for the lack of correlation between the mouse and human phenotypes with CHI are not entirely clear. Natural history of CHI There is now some evidence to show that the natural history of CHI is a slow progressive loss of b-cell function, and this may be due to the increased b-cell apoptosis.46 It has been shown that patients with CHI who have had their pancreas removed, and who have mutations in the ABCC8 (SUR1) gene, have increased number of apoptotic cells in focal lesions.47 The precise mechanism of apoptosis is unclear but may be related to the increased intracellular calcium concentrations as a result of unregulated calcium entry.48 A unique example of the natural history of CHI is illustrated by the dominant heterozygous missense mutation (E1506K) in the sulphonylurea receptor ABCC8 gene in a large Finnish family.49 In the infancy period heterozygous E1506K carriers of this mutation have a mild form of CHI which is responsive to diazoxide.49 In early adulthood this mutation causes loss of insulin secretory capacity with glucose intolerance, and then in middle age diabetes mellitus develops. The underlying mechanism of how this mutation causes the development of diabetes mellitus over time is thought to be related to apoptosis, but this is unproven. Neurological outcome Neurological outcome depends on age at presentation. Although there are no studies documenting the frequency, severity, and duration of persistent hyperinsulinaemic hypoglycaemia which results in brain injury, neonates with medically unresponsive CHI are at an increased risk of neurological damage.3 Other factors which increase the risk of brain damage include delay in establishing the diagnosis and the need for surgical treatment.50 There seems to be no difference in neurological outcome between diffuse or focal CHI presenting in the neonatal period.3 Conclusions CHI is a cause of persistent and recurrent hypoglycaemia in the newborn period. In order to prevent brain damage the hypoglycaemia must be managed promptly. The differentiation of focal and Congenital hyperinsulinism diffuse disease is now imperative as management is different for the two forms. Evidence is emerging that some forms of CHI may be associated with slow progressive loss of b-cell function. Mice knockout models of CHI are phenotypically different from the human phenotype. Practice points CHI is a cause of persistent hypoglycaemia in the neonatal period The biochemical profile is one of hypofattyacidaemic, hypoketotic hyperinsulinaemic hypoglycaemia (no alternative substrates for the brain to use); hence management of the hypoglycaemia must be prompt in order to avoid brain damage The surgical management of the focal form of CHI is different from that of the diffuse form Infants with CHI should be referred to a centre that has the expertise and experience to manage these patients Research directions Understanding the genetic basis of CHI in the remaining 50% of patients Understanding the mechanisms leading to transient hyperinsulinism Developing new therapeutic interventions for children with diffuse disease so that near-total pancreatectomy can be avoided Understanding why mouse models of CHI are phenotypically different from the human phenotype Acknowledgements Research at the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust benefits from research and development funding received from the NHS Executive. References 1. Laidlaw GF. Nesidioblastoma, the islet tumor of the pancreas. Am J Pathol 1938;14:125e34. 375 2. Rahier J, Guiot Y, Sempoux C. Persistent hyperinsulinaemic hypoglycaemia of infancy: a heterogeneous syndrome unrelated to nesidioblastosis. Arch Dis Child Fetal Neonatal Ed 2002 Mar;82(2):F108e12. 3. Menni F, de Lonlay P, Sevin C, Touati G, Peigne C, Barbier V, et al. Neurologic outcomes of 90 neonates and infants with persistent hyperinsulinemic hypoglycemia. Pediatrics 2001; 107:476e9. 4. Dunne MJ, Cosgrove KE, Shepherd RM, Aynsley-Green A, Lindley KJ. Hyperinsulinism in infancy: from basic science to clinical disease. Physiol Rev 2004 Jan;84(1):239e75. 5. Aynsley-Green A, Hussain K, Hall J, Saudubray JM, NihoulFekete C, De Lonlay-Debeney P, et al. The practical management of hyperinsulinism in infancy. Arch Dis Child Fetal Neonatal Ed 2000;82:F98e107. 6. De Lonlay P, Fournet JC, Touati G, Groos MS, Martin D, Sevin C, et al. Heterogeneity of persistent hyperinsulinaemic hypoglycaemia. A series of 175 cases. Eur J Pediatr 2002 Jan;161(1):37e48. 7. Brunelle F, Negre V, Barth MO, Fekete CN, Czernichow P, Saudubray JM, et al. Pancreatic venous samplings in infants and children with primary hyperinsulinism. Pediatr Radiol 1989;19:100e3. 8. De Lonlay-Debeney P, Poggi-Travert F, Fournet JC, Sempoux C, Viei CD, Brunelle F, et al. Clinical features of 52 neonates with hyperinsulinism. N Engl J Med 1999 Apr 15; 340(15):1169e75. 9. Thomas PM, Cote GJ, Wohllk N, Haddad B, Mathew PM, Rabl W, et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science 1995 Apr 21;268(5209):426e9. 10. Nestorowicz A, Wilson BA, Schoor KP, Inoue H, Glaser B, Landau H, et al. Mutations in the sulphonylurea receptor gene are associated with familial hyperinsulinism in Ashkenazi Jews. Hum Mol Genet 1996 Nov;5(11):1813e22. 11. Glaser B, Kesavan P, Heyman M, Davis E, Cuesta A, Buchs A, et al. Familial hyperinsulinism caused by activating glucokinase mutation. N Engl J Med 1998 May 7;338(19):226e30. 12. Stanley CA, Lieu YK, Hsu BY, Burlina AB, Greenberg CR, Hopwood NJ, et al. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med 1998 May 7;338(19):1352e7. 13. Clayton PT, Eaton S, Aynsley-Green A, Edginton M, Hussain K, Krywawych S, et al. Hyperinsulinism in shortchain L-3-hydroxyacyl-CoA dehydrogenase deficiency reveals the importance of beta-oxidation in insulin secretion. J Clin Invest 2001 Aug;108(3):457e65. 14. Hussain K, Aynsley-Green A. Management of hyperinsulinism in infancy and childhood. Ann Med 2000;32:544e51. 15. Hussain K, Aynsley-Green A. Hyperinsulinaemic hypoglycaemia in preterm neonates. Arch Dis Child Fetal Neonatal Ed 2004 Jan;89(1):F65e7. 16. Collins JE, Leonard JV. Hyperinsulinism in asphyxiated and small for dates infants with hypoglycaemia. Lancet 1984;ii: 311e3. 17. Mehta A, Hussain K. Transient hyperinsulinism associated with macrosomia, hypertrophic obstructive cardiomyopathy and hepatomegaly. Arch Dis Child 2003 Sep;88(9):822e4. 18. de Lonlay P, Seta N, Barrot S, Chabrol B, Drouin V, Gabriel BM, et al. A broad spectrum of clinical presentations in congenital disorders of glycosylation I: a series of 26 cases. J Med Genet 2001 Jan;38(1):14e9. 19. Meissner T, Rabl W, Mohnike K, Scholl S, Santer R, Mayatepek E. Hyperinsulinism in syndromal disorders. Acta Paediatr 2001 Aug;90(8):856e9. 20. Lindley KJ, Knafelz D, St Louis D, Dunne MJ, AynsleyGreen A, Milla PJ, et al. Surface electrogastrographic 376 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. evidence of foregut dysmotility in persistent hyperinsulinaemic hypoglycaemia of infancy (PHHI). J Physiol (Lond) 1997;499:108. Hussain K, Hindmarsh P, Aynsley-Green A. Neonates with symptomatic hyperinsulinaemic hypoglycaemia generate inappropriately low serum cortisol counter-regulatory hormonal responses. J Clin Endocrinol Metab 2003 Sep;88(9): 4342e7. Levitt-Katz LE, Satin-Smith M, Collet-Solberg P, Thornton PS, Baker L, Stanley CA, et al. Insulin like growth factor binding protein-1 levels in the diagnosis of hypoglycaemia caused by hyperinsulinism. J Pediatr 1997;131: 193e9. Finegold DN, Stanely CA, Baker L. Glycaemic response to glucagon during fasting hypoglycaemia: an aid in the diagnosis of hyperinsulinism. J Pediatr 1980;96:257. Malaisse WJ, Sener A, Herchuelz, Hutton JC. Insulin release: the fuel hypothesis. Metabolism 1979;28:373e86. Dunne MJ, Peterson OH. Potassium selective ion channels in insulin-secreting cells: physiology, pharmacology and their role in stimulusesecretion coupling. Biochim Biophys Acta 1991 Mar 7;1071(1):67e82 [Review]. Inagaki N, Gonoi T, Clement JPT, Namba N, Inazawa J, Gonzalez G, et al. Reconstitution of KATP: an inward rectifier subunit plus the sulfonylurea receptor. Science 1995 Nov 17;270(5239):1166e70. Straub SG, Cosgrove KE, Ammala C, Shepherd RM, O’Brien RE, Barnes PD, et al. Hyperinsulinism of infancy: the regulated release of insulin by KATP channel-independent pathways. Diabetes 2001 Feb;50(2):329e39. Kane C, Shepherd RM, Squires PE, Johnson PR, James RF, Milla PJ, et al. Loss of functional KATP channels in b-cells causes persistent hyperinsulinaemic hypoglycaemia on infancy. Nat Med 1996;2:1344e7. Molven A, Matre GE, Duran M, Wanders RJ, Rishaug U, Njolstad PR, et al. Familial hyperinsulinemic hypoglycemia caused by a defect in the SCHAD enzyme of mitochondrial fatty acid oxidation. Diabetes 2004;53:221e7. Stanley CA. Hyperinsulinism in infants and children. Pediatr Clin North Am 1997 Apr;44(2):363e74 [Review]. Barnes PD, O’Brien RE, Cosgrove KE, Abdel-Wahab YHA, Flatt PR, Aynsley-Green A, et al. The hyperglycaemic effects of hydrochlorothiazide involve activation of potassium channels in insulin secreting cells. Arch Dis Child 2000; 82(Suppl. 1):P24. Lindley KJ, Dunne MJ, Kane C, Shepherd RM, Squires PE, James RF, et al. Ionic control of the b-cell function in nesidioblastosis, a possible role for calcium channel blockade. Arch Dis Child 1996 May;74(5):373e8. Shanbag P, Pathak A, Vaidya M, Shahid SK. Persistent hyperinsulinemic hypoglycemia of infancy-successful therapy with nifedipine. Indian J Pediatr 2002 Mar;69(3): 271e2. Moens K, Berger V, Ahn JM, Van Schravendijk C, Hruby VJ, Pipeleers D, et al. Assessment of the role of interstitial glucagon in the acute glucose secretory responsiveness of in situ pancreatic beta-cells. Diabetes 2002;51:669e75. Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol 1999;20:157e98. Hussain K, Cosgrove KE, Shepherd RM, Chapman JC, Swift SM, Smith VV, et al. Uncontrolled insulin secretion from a childhood pancreatic b-cell adenoma is not due to the functional loss of ATP-sensitive potassium channels. Endocr Relat Cancer 2002;9(4):221e6. K. Hussain 37. De Lonlay P, Fournet JC, Rahier J, Gross-Morand MS, PoggiTravert F, Foussier V, et al. Somatic deletion of the imprinted 11p15 region in sporadic persistent hyperinsulinemic hypoglycemia of infancy is specific of focal adenomatous hyperplasia and endorses partial pancreatectomy. J Clin Invest 1997 Aug 15;100(4):802e7. 38. Verkarre V, Fournet JC, Delonlay P, Gross-Morand MS, Devillers M, Rahier J, et al. Paternal mutation of the sulphonylurea receptor (SUR 1) gene and maternal loss of the 11p15 imprinted genes lead to persistent hyperinsulinism in focal adenomatous hyperplasia. J Clin Invest 1998; 102:1286e91. 39. Leibowitz G, Glaser B, Higazi AA, Salamah M, Cesari E, Landau H, et al. Hyperinsulinaemic hypoglycaemia of infancy (nesidioblastosis) in clinical remission: high incidence of diabetes mellitus and persistent b-cell dysfunction at long term follow up. J Clin Endocrinol Metab 1995; 80:386e92. 40. Dubois J, Brunelle F, Touati G, Sebag G, Nuttin C, Thach T, et al. Hyperinsulinism in children: diagnostic value of pancreatic venous sampling correlated with clinical, pathological and surgical outcome in 25 cases. Pediatr Radiol 1995;25(7):512e6. 41. Abernethy LJ, Davidson DC, Lamont GL, Shepherd RM, Dunne MJ. Intra-arterial calcium stimulation test in the investigation of hyperinsulinaemic hypoglycaemia. Arch Dis Child 1998 Apr;78(4):359e63. 42. Otonkoski T, Veijola R, Huopio H, Nanto-Salonen K, Tapanainen P, delonlay P, et al. Diagnosis of focal persistent hyperinsulinism of infancy with 18F-fluoro-L-dopa PET. Horm Res 2003;60(Suppl. 2):P2. 43. Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A 1998;95:10402e6. 44. Miki T, Tashiro F, Iwanaga T, Nagashima K, Yoshitomi H, Aihara H, et al. Abnormalities of pancreatic islets by targeted expression of a dominant-negative KATP channel. Proc Natl Acad Sci U S A 1997;94:11969e73. 45. Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, Bryan J. Sur1 knockout mice. A model for KATP channel-independent regulation of insulin secretion. J Biol Chem 2000;275(13): 9270e7. 46. Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 2000 Aug;49(8):1325e33. 47. Glaser B, Ryan F, Donath M, Landau H, Stanley CA, Baker L, et al. Hyperinsulinism caused by paternal-specific inheritance of a recessive mutation in the sulfonylurea-receptor gene. Diabetes 1999 Aug;48(8):1652e7. 48. Efanova IB, Zaitsev SV, Zhivotovsky B, Kohler M, Efendic S, Orrenius S, et al. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells. A process dependent on intracellular Ca2C concentration. J Biol Chem 1998 Dec 11;273(50): 33501e7. 49. Huopio H, Otonkoski T, Vauhkonen I, Reimann F, Ashcroft FM, Laakso M, et al. A new subtype of autosomal dominant diabetes attributable to a mutation in the gene for sulfonylurea receptor 1. Lancet 2003 Jan 25;361(9354): 301e7. 50. Meissner T, Brune W, Mayatepek E. Persistent hyperinsulinaemic hypoglycaemia of infancy: therapy, clinical outcome and mutational analysis. Eur J Pediatr 1997;156:754e7.