* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download evaluation for flushing, safety and tolerability of niacin
Survey
Document related concepts
Effect size wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Psychopharmacology wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug design wikipedia , lookup
Prescription costs wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Transcript
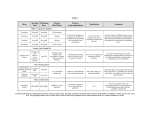
EVALUATION FOR FLUSHING, SAFETY AND TOLERABILITY OF NIACIN (N) IN COMBINATION WITH 40 MG OF OXYCODONE (O) D. R. Jasinski; Johns Hopkins, Baltimore, MD ABSTRACT METHODS Background: The objectives were 1) to determine the dose response, safety, tolerability and abuse for N flushing in opioid abusers when administered with O and 2) to evaluate the effect of food on N flushing. Methods: This is a double-blind, randomized, crossover study in 25 subjects who received drug every 48 hours. The dosing sequence included N (0, 240, 480, and 600 mg) with O while the subjects were fasted. On Day 9, 600 mg N with O was administered following a standardized high-fat breakfast. Each dosing day, vital signs, subjective and behavioral effects were assessed at -0.5 and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, and 12 hours. Subjective changes were measured with the Drug Rating Questionnaire (subjects)-DRQS and a short form of the Addiction Research Inventory (ARCI), at completion, subjects completed the Treatment Enjoyment Assessment Questionnaire. The maximum scale response to the question “Do you dislike the drug effect you are feeling now?” or Disliking Score in the DRQS is the primary efficacy variable. Results: 25 subjects (3 female and 22 male) entered but 1 male subject withdrew. Analysis was for the 24 completers. N does not alter the time course of subjective effects or pupillary constriction of O. After the high fat meal, the peak subjective effects and pupillary constriction occur at 3 to 4 hours indicating a delay in the absorption of O. In the fasting state, all 3 doses of N added to O produced significant Disliking Scores; however, the linear regression across dose is not significant. The high fat meal eliminated the N effect. No other subjective measure was significantly affected by the N. N produced a dose related attenuation of the pupillary constriction, diastolic increase and probably systolic blood pressure increase produced by O. Therewere no serious adverse events. Conclusion: N to O in a minimal ratio of 30 mg N to 5 mg O is aversive when compared to O. The addition of N does not alter the safety profile of O alone. STUDY OBJECTIVES PHYSIOLOGIC MEASURES 1. Determine the dose response for niacin-induced flushing when niacin is combined with 40 mg oxycodone HCl 2. Evaluate the safety and tolerability of such niacin–induced flushing following varying combined niacin doses 3. Confirm the strength of niacin to use in a formulation ofoxycodone 4. Determine whether the flushing by niacin is sufficient to deter abuse 5. Evaluate the effect of food on flushing from the niacin and oxycodone flushing 1. Pupil Size 2. Systolic and Diastolic Blood Pressure 3. Pulse Rate 4. Oral Temperature 5. Respiratory Rate BACKGROUND Abusers tamper with prescription opioids such as oxycodone for intravenous injection; 1. intranasal snorting and oral ingestion of excessive quantities 2. Acura Pharmaceuticals, Inc has developed Acurox™ (oxycodone HCl and niacin) Tablets to reduce such tampering a. Gel forming polymer to reduce extraction b. A mucosal irritating surfactant to discourage inhalation c. Niacin (Vitamin B3) to induce flushing at levels exceeding the recommended dosage. patientresearch care STUDY DESIGN 1. 21 male and 3 female opioid abusers on a residential unit 2. Double-blind, randomized, cross-over design 3. Five single doses of drug every 48 hours over 9 days 4. Days 1, 3,5,7 a. niacin 0, 240, 480, and 600 mg in combination with oxycodone HCL 40 mg to fasting subjects b. given in random order according to 6 4X4 Latin Squares 5. Day 9 a. niacin 600 mg in combination with oxycodone HCl 40 mg b. administered orally after a standardized fatty meal RESULTS Dunnett t 1. DRQS and Physiologic Measures a. Before dosing and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, and 12 hours after dosing b. Maximum change from baseline used as measure of drug effect 2. ARCI a. 0.5, 1, 1.5, 2, 3, 4, 5, 6, and 12 hours after dosing b. Maximum response used as measure of drug effect 3. TEAQ a. 24 hours after last drug administration b. Choice of one treatment to take again (J) Drug Dependent Variable Condition Condition Do you feel a drug 240 mg 0 mg effect now? niacin niacin 480 mg 0 mg niacin niacin 600 mg 0 mg niacin niacin 240 mg 0 mg effect you are feeling niacin niacin now? 480 mg 0 mg niacin niacin 600 mg 0 mg niacin niacin Do you dislike the 240 mg 0 mg drug effect you are niacin niacin feeling now? 480 mg 0 mg niacin niacin 600 mg 0 mg niacin niacin Morphine Benzedrine 240 mg niacin 480 mg 0 mg niacin niacin 600 mg 0 mg niacin niacin Pentobarbital 240 mg 0 mg Chlorpromazine niacin niacin Alcohol Group Scale 480 mg 0 mg niacin niacin 600 mg 0 mg niacin niacin 240 mg 0 mg niacin niacin 480 mg 0 mg niacin niacin 600 mg 0 mg niacin niacin LSD Specific Scale * Difference Std. Error (I-J) 0 mg niacin Group Scale Sig. 1.8 1.7 0.31 1.2 1.7 0.46 3.9* 1.7 0.03 -0.8 1.8 0.88 -1.1 1.8 0.92 -1.2 1.8 0.92 5.1 2.4 0.05 Dependent Variable (I) Drug Condition (J) Drug Condition Pupil Size 240 mg niacin 0 mg niacin 480 mg niacin 0 mg niacin 600 mg niacin 0 mg niacin 240 mg niacin 0 mg niacin 480 mg niacin 0 mg niacin 600 mg niacin 0 mg niacin Diastolic Blood Pressure * 6.3 * 2.4 0.01 9.0 * 2.4 0.00 -0.2 0.7 0.99 0.1 0.7 1.00 0.0 0.7 1.00 0.0 0.6 1.00 * 0.6 0.04 0.6 0.10 -1.4 -1.2 0.6 0.5 Mean Difference Std. Error (I-J) .01 .152 .02 .152 * .15 .39 .17 1.515 -2.08 1.515 * 1.51 -4.21 0.5 0.67 0.7 0.5 0.38 (I) Drug Condition (J) Drug Condition Do you dislike the drug effect you are feeling now? 600 mg niacin 0 mg niacin 600 mg niacin (with high fat meal) 0 mg niacin Diastolic Blood Pressure 600 mg niacin 0 mg niacin 600 mg niacin (with high fat meal) 0 mg niacin 600 mg niacin 0 mg niacin 600 mg niacin (with high fat meal) 0 mg niacin Pupil Size * 9.0 * 1.6 -4.2 2.6 0.751 * 0.3 0.4 1.7 0.029 1.7 0.972 * -0.2 2.6 0.002 0.1 0.017 0.1 0.192 The mean difference is significant at the .05 level. Effects of High Fat Meal on Niacin Effects 1. High fat meal eliminated the niacin effect 2. High fat meal also delayed the time to peak for oxycodone effects TREATMENT ENJOYMENT ASSESSMENT QUESTIONAIRRE 0.49 0.5 Mean Difference Std. Error Sig. (I-J) Dependent Variable The mean difference is significant at the .05 level. Choice Percent None of the drugs 6 25.0 0 mg niacin + 40 mg oxycodone 2 8.3 240 mg niacin + 40 mg oxycodone 4 16.7 480 mg niacin + 40 mg oxycodone 1 4.2 600 mg niacin + 40 mg oxycodone 1 4.2 600 mg niacin + 40 mg oxycodone (with high fat meal) 10 41.7 Total 24 100.0 The mean difference is significant at the .05 level. TREATMENT ENJOYMENT ASSESSMENT QUESTIONAIRRE 1. Highest frequency is the combination with 600 mg of niacin 2. High fat and placebo total 12 of 24 subjects 3. High fat meal was the last treatment condition thereby limiting interpretation Analysis • General Linear Model Analysis of Variance for six 4X4 balanced Latin Squares 1. Drug Rating Questionnaire Subjects (DRQS) • Individual maximum response a. Do you feel a drug effect now? or change from baseline used b. Do you like the drug effect you are feeling now? as measure of drug effect for c. Do you dislike the drug effect you are feeling now? ANOVA 2. Addiction Research Center Inventory (ARCI) • Mean responses for niacin a. Morphine Benzedrine Group Scale (MBG) dose compared with Dunnet b. LSD Specific Scale (LSD) t using error mean square from c. Pentobarbital Chlorpromazine Alcohol Group ANOVA Scale (PCAG) • Mean response or mean 3. Treatment Enjoyment Assessment Questionnaire maximum change compared a. Which of five treatments would like to take again? graphically Effects of High Fat Meal on Niacin Effects Dunnett t (2-sided) Dunnet t (2- sided) Mean (I) Drug Do you like the drug OBSERVATIONS Effect of Niacin Compared to Placebo on Physiologic Measures Effect of Niacin Compared to Placebo on Subjective Measures SUBJECTIVE MEASURES CONCLUSION Effects of Niacin on Oxycodone Subjective Effects 1. All doses of niacin added to oxycodone produced significant disliking scores. 2. Niacin disliking effect tended to be dose related (p=.108) 3. No other statistically significant changes in subjective effects 1. Niacin in the proposed ratio of 30 mg to 5 mg oxycodone is aversive 2. Niacin does not alter the safety profile of oxycodone a. Physiologic changes from largest dose niacin clinically insignificant b. No significant differences in adverse event profile Effects of Niacin on Oxycodone Physiologic Effects 1. At 600 mg attenuated the pupillary constriction of oxycodone 2. At 600 mg attenuated the diastolic blood pressure increase of oxycodone THIS STUDY SUPPORTED BY ACURA PHARMACEUTICALS, INC Media Services at JHBMC 410.550.0677