* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Mitral Clip - Society of Cardiovascular Anesthesiologists
Survey
Document related concepts
Cardiovascular disease wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Coronary artery disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Rheumatic fever wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Pericardial heart valves wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Artificial heart valve wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Transcript
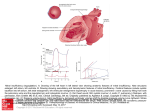
The Mitral Clip: What to Expect and What to do When Things Go Wrong Fabio Guarracino MD Head, Department of Anesthesia and Intensive Care Medicine Director of Cardiothoracic Anesthesia and Intensive Care University Hospital, Pisa, Italy Introduction Mitral regurgitation (MR) is one of the most common valve disease. The degree of regurgitation, mild, moderate or severe, and the presence of symptoms or signs of left ventricular dysfunction guide the therapeutic strategies. Surgery is strongly recommended in symptomatic patients presenting moderate to severe and severe MR . Despite the lack of randomized control trials, it is widely accepted that surgical repair, when feasible, is the treatment of choice in patients with moderate to severe MR because valve repair is associated with a lower perioperative mortality, improved survival, better preservation of post-operative left ventricular function, and lower longterm morbidity if compared with valve replacement. In early nineties the edge-to-edge repair has been introduced as a surgical technique for the treatment of MR . In this technique, the edges of both the middle scallop of the posterior and anterior leaflets are sutured together, so creating a point of permanent coaptation. This results in a double-orifice valve, which resembles a typical “eight” shape. Nowadays the development of very sophisticated new technology has allowed high-risk patients to be adequately treated for symptomatic severe MR The MitraClip® system (Evalve Inc., Menlo Park, California) is an emergent alternative to surgery in the MR repair. This is a percutaneous edge-to-edge repair of the mitral valve mimicking the Alfieri surgical procedure. A clip is positioned between the anterior and posterior leaflet to reduce the valve regurgitation. Clinical Indications to edge-to-edge clip repair: Moderate to severe or severe chronic mitral valve regurgitation and symptomatic with LVEF <25% and LVID-s <55 mm or asymptomatic with 1 or more of the following: LVEF >25% to 60% LVID-s >40 to 55 mm New onset of atrial fibrillation Pulmonary hypertension defined as pulmonary artery systolic pressure >50 mm Hg at rest or >60 mm Hg with exercise. Another important criterion of inclusion is the possibility to perform an adequate trans-septal puncture. Exclusion criteria for Mitralclip procedure: • Recent myocardial infarction • Patients with significant MR with rheumatic disease, endocarditis • Patients who presented abnormal leaflets thickness or calcifications • Patients with functional MR with a coapting surface length < 2mm and/or a coaptation depth >11 mm ; • Patients with degenerative MR with a flail height of >10 mm and a flail width of 15 mm. • Mitral valve orifice area >4 cm2 The procedure The MitraClip System includes a MitraClip device, a 24-F Steerable Guide Catheter (SGC) and a Clip Delivery System (CDS). The Clip is pre-assembled to the tip of the disposable delivery catheter. Opening, closing, locking, and detaching the clip are all controlled by the delivery catheter handle mechanisms, which is firmly lodged on a metal sterilized external support placed outside of the patient, on the bottom of a small table above the upper leg. The procedure is performed under general anesthesia and TEE monitoring. Invasive arterial pressure is monitored through radial or femoral artery, a central venous catheter is placed in the right internal jugular vein or in a subclavian vein. The right femoral vein is cannulated with a 7 F introducer sheath, and a baseline right heart catheterization is performed. Then the 7 fr introducer is exchanged with an 8-F Mullins sheath (S. Jude Medical, MN, USA) over a 0.32 guidewire, and a trans-septal puncture is performed using a Brockenbrough needle under TOE guidance. This is a critical point of the procedure, because the puncture has to be located in the posterior-superior part of the interatrial septum in order to obtain enough room in the left atrium for a safe and optimal orientation of the steerable distal part of the CDS. Once the left atrium is entered with the 8 F sheath, the left pulmonary vein is cannulated using a 6-F multipurpose catheter (MPA2, Cordis, Johnson & Johnson,Miami, Florida) and a 260 cm Amplatz Super stiff guidewire is left in place. After administration of 100 IU/kg of unfractioned heparin, the 24-F SGC is introduced in the left atrium and the dilator is carefully and slowly retrieved for avoiding vacuum air-bubbles. The CDS is then advanced in the left atrium and the distal steerable part is manipulated in the atrium for obtaining a perpendicular and central position with respect to the mitral valve leaflets coaptation line . The correct trajectory of the clip and the perpendicularity of the two arms with respect to the mitral leaflet coaptation line are checked using three echocardiographic views: 1) the three chamber in where the left atrium, left ventricle and aortic root are visualized; 2) the perpendicular dual chamber, in where both left atrium and ventricle are obtained, and 3) the trans-gastric short axis view to visualize the coaptation line of mitral leaflets. Once the system has been aligned, the clip with opened arms is advanced into the left ventricle and under TEE guidance the arms grasp the leaflets. When a double orifice has been created and the echocardiography confirms the regurgitation reduction and the optimal and stable grasp of both leaflets, there are two options: if the position is suboptimal, the clip can be reopened and repositioned; if the result is good and the grasp is stable, the clip arms are closed, locked and detached and the SGC and CDS are withdrawn. The expected result and the possible complications The expected result of the procedure consists of MR reduction < 2+. This is usually obtained with one clip implantation. In some cases a second clip can be needed. This usually occurs when TEE evaluation reveals a residual MR > 2+ after the first clip implantation. A successfull Mitraclip procedure usually shows the following acute result: 1. MR < 2+ 2. Improved cardiac output 3. Reduced wedge pressure and LVED pressure However complications are possible, leading to intraoperative decision-making aimed at solving the acute problem. The main complications can be mechanical and functional. The former are: 1. Clip entrapment by cordae within the LV 2. Partial clip detachment 3. Pericardial tamponade 4. Atrial septal rupture The functional complication can be: 1. MR > 2+ after 2/3 clips implantation 2. Acute LV dysfunction due to abrupt increase of LV afterload after the MR reduction In all these situations TEE plays a crucial role in guiding the decision-making process, and the 3D TEE adds a great advantage in supporting the procedure both during uneventful and complicated cases. What to do when things go wrong. In case of clip interaction with cordae or subvalvular apparatus the clip arms are inverted and careful maneuvers are needed to retract the clip into the left atrium under TEE and fluoroscopy guidance. When partial clip detachment occurs, a second clip is needed in order to stabilize the leaflets and allow for coaptation. In some circumstances surgery may be considered. Pericardial tamponade needs TEE assisted pericardiocentesis. The decision-making can be more difficult in case of MR > 2+. If hemodynamics is stable, the decision is usually to go on with medical treatment and see over the next months. However in some cases MR can be severe, even worse than basal MR, due to MV distortion induced by the clip implantation. In such cases the surgical option can be discussed. The septal rupture is rare, but a significant shunt across the atrial septum may require correction by Amplatzer septal closure device implantation. When the acute reduction of MR causes an abrupt increase of LV afterload, an acute LV failure can occur, especially in patients with ischemic/functional MR and depressed LV function. In such cases inotropic support can be needed and mechanical ventilation can prolong beyond the procedure, therefore requiring postoperative care in the ICU. Conclusion. Correction of the mitral insufficiency, either degenerative either ischemic/functional, with MitraClip system is an emergent technique. This procedure is reported to be feasible in patients with high risk profile. Perioperative management requires team approach, and the same cautions as in the surgical setting, including postoperative monitoring in ICU. The role of TEE is more complex than in surgical mitral repair due to the guiding role it plays in the crucial phases of the procedure. The live 3D technology implemented in the transoesophageal transducers provides a useful tool to support the Mitraclip procedure by better guiding the device positioning and orientation, improving the grasping phase and checking the final result. The procedural success consists in MR reduction <2+ and occurs in the majority of cases. However things can go wrong due to mechanical and functional complications. In such cases a cautious, team based, case-by-case approach is needed to manage the complicated course. Suggested readings • Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, Nataf P, Tornos P, Torracca L, Wenink A; Task Force on the Management of Valvular Hearth Disease of the European Society of Cardiology; ESC Committee for Practice Guidelines. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230-68. • American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons, Bonow RO, Carabello BA, Kanu C, de Leon AC Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84-231. • Maisano F, Torracca L, Oppizzi M, Stefano PL, D'Addario G, La Canna G, Zogno M, Alfieri O. The edge-to-edge technique: a simplified method to correct mitral insufficiency. Eur J Cardiothorac Surg. 1998 Mar;13(3):240-5. • Alfieri O, Maisano F, De Bonis M, Stefano PL, Torracca L, Oppizzi M, La Canna G. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg. 2001;122:674-81 • Feldman T, Kar S, Rinaldi M, Fail P, Hermiller J, Smalling R, Whitlow PL, Gray W, Low R, Herrmann HC, Lim S, Foster E, Glower D; EVEREST Investigators. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol. 2009;54:686-94. • Biner S, Perk G, Kar S, Rafique AM, Slater J, Shiota T, Hussaini A, Chou S, Kronzon I, Siegel RJ. Utility of combined two-dimensional and three-dimensional transesophageal imaging for catheter-based mitral valve clip repair of mitral regurgitation. J Am Soc Echocardiogr. 2011 Jun;24(6):611-7. Epub 2011 Mar 23. • Altiok E, Becker M, Hamada S, Reith S, Marx N, Hoffmann R. Optimized guidance of percutaneous edge-to edge repair of the mitral valve using real-time 3-D transesophageal echocardiography. Clin Res Cardiol. 2011 Aug;100(8):675-81. • Ciobanu A, Bennett S, Azam M, Clark A, Vinereanu D. Incremental value of three-dimensional transoesophageal echocardiography for guiding double percutaneous MitraClip ® implantation in a 'no option' patient. Eur J Echocardiogr. 2011 Feb;12(2):E11. • Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L; EVEREST II Investigators. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011 Apr 14;364(15):1395-406.