* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cardiac Resynchronization: The Flow of Activation Sequence
Heart failure wikipedia , lookup
Cardiac surgery wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Myocardial infarction wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Electrocardiography wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Heart arrhythmia wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
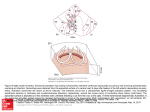
JACC: CARDIOVASCULAR IMAGING VOL. 6, NO. 8, 2013 ª 2013 BY THE AMERICAN COLLEGE OF CARDIOLOGY FOUNDATION PUBLISHED BY ELSEVIER INC. ISSN 1936-878X/$36.00 http://dx.doi.org/10.1016/j.jcmg.2013.07.002 iVIEW EDITOR’S PAGE Cardiac Resynchronization: The Flow of Activation Sequence Partho P. Sengupta, MD,* Christopher M. Kramer, MD,y Jagat Narula, MD, PHD* A lthough cardiac resynchronization therapy (CRT) has become a mainstay of therapy for systolic heart failure refractory to medical management for over a decade, more than 30% of CRT recipients do not respond clinically to the intervention. Ideally, cardiologists should be able to predict those who might not respond to CRT in advance and withhold these costly devices in likely nonresponders. However, because of conflicting results and difficulties in mapping the 3-dimensional sequence of left ventricular (LV) electromechanical activation, an effective approach to quantifying dyssynchrony is yet to be established. The normal electromechanical activation sequence of the LV develops from a close interaction of the specialized conduction system with the myocyte architecture. Nearly the entire LV endocardial layer is directly activated within approximately 40 ms by Purkinje cells (Fig. 1) (1). The epicardial layer, which is void of Purkinje cells, experiences delayed activation through cell-to-cell propagation of electrical activity. Recently, Ramanathan et al. (2) noninvasively studied the normal epicardial activation sequence in intact healthy adults. The endocardial-to-epicardial activation of the LV free wall occurred with a breakthrough seen first over the LV apex. Subsequent apex-to-base spread of activation was associated with the latest activation occurring in the LV posterolateral basal region. The timing of mechanical activation follows the electrical sequence, but the transmural speed of myofiber shortening (0.25 m/s) lags the speed of electrical conduction (0.49 m/s) (3). This delay is related to the development of a transient stretch in the late-activated epicardial regions of the left ventricle. This stretch of the late-activated regions of the left ventricle is due to shortening of early-activated regions and a steep rise in LV pressure in the absence of a change in LV volume during isovolumic contraction. This stretch (of From the *Icahn School of Medicine at Mount Sinai, New York, New York; and the yUniversity of Virginia, Charlottesville, Virginia. up to 10%) of the late depolarized myofibers (which also accounts for transient clockwise rotation of the apex during isovolumic contraction) is helpful in generating more shortening during ejection (with subsequent counterclockwise rotation of the apex during ejection). The pre-ejection stretch increases the sarcomeric length in subepicardial myofibers and enhances shortening strains per the Frank-Starling mechanism (4,5). The reciprocal shortening and stretching of the LV wall also alters the LV geometry, which helps preferential streaming of blood flow toward the LV outflow (5). The specific patterns of delay in LV activation in cardiomyopathic patients who benefit from CRT may also be better understood by considering the sequence of electromechanical coupling and the overall effects on the timing of LV contraction and blood flow. First, electrical activation delays that result from a transmural pattern of block (particularly from the left bundle) may be more amenable to CRT. Recent observations in the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy study suggest that the benefit of CRT in patients with class I and II heart failure was confined to those who had classic complete left bundle branch block (LBBB), whereas those with a non-LBBB configuration did not derive as much benefit (6). Thus, there has been an increase in the importance of separating LBBB from diffuse intraventricular conduction disturbances. Auricchio et al. (7) used 3-dimensional mapping systems to study the sequence of LV activation in patients with heart failure with LBBB QRS configuration during intrinsic rhythm (as also during asynchronous pacing). They demonstrated a U-shaped conduction pattern in the activation sequence of the left ventricle in patients with LBBB. Noncontact mapping showed that the activation wave front could not cross directly to the lateral wall from the anterior region. Instead, this wave front reached the lateral or posterolateral regions by propagating from the site of earliest septal LV breakthrough, inferiorly around the apex and across the inferior wall (Fig. 1). This JACC: CARDIOVASCULAR IMAGING, VOL. 6, NO. 8, 2013 AUGUST 2013:924–6 U-shaped block, however, was altered on asynchronous pacing. Moreover, the presence of near normal amplitude on unipolar and bipolar electrocardiography excluded the presence of scar tissue, and the presence of fractionated electrocardiographic results suggested that the block was functional and resulted from the nonuniform conduction within the transmural layers of the myocardium. Interestingly, the study by Sohal et al. (8) in this issue of iJACC also revealed the presence of a U-shaped mechanical activation block between the septum and lateral wall, and this pattern of block was commonly associated with response to CRT. Just as the transmural electrical activation delay manifests on the surface as a U-shaped block, the observations regarding mechanical activation delay between the septum and the lateral wall may only signify a disruption of LV mechanics between the transmural layers. Normal radial LV wall thickening during ejection requires mechanical interaction of both the early-activated subendocardial and the lateactivated subepicardial regions. Activity of both regions needs to be developed in concert for optimal wall thickening; the subendocardial myofibers must further thicken and slide inward, and this inward slippage (shearing) accounts for the larger radial thickening strains (>40%) and segmental volume change (>60%) despite relatively small myocyte contraction developed within the layers (about 15%). Thus, the identification of transmural functional block rather than segmental or regional blocks may better identify patients likely to benefit from CRT. Transmural mechanical activation delay may also impair diastolic function, because LV systolic and diastolic performances are closely coupled. In normal subjects, the untwisting and recoil of the LV wall in early diastole releases elastic energy stored by the preceding systolic deformation. This creates early diastolic suction with the formation of a vortex ring at the mitral valve tip (9). The vortex rings store part of the kinetic energy of the entering flow into its rotary motion and help redirect the flow toward the outflow tract. A recent study showed that deactivation of CRT results in a delayed onset of LV filling in early diastole, with a delayed onset of blood flow vortex formation (10). A weaker early diastolic vortex further restricted the energy transfer from diastole to systole, impeding the timely onset of LV ejection. Thus, the normal sequence of electromechanical activation can also be valued in synchronizing an optimal transfer of kinetic energy from diastole into systole. In summary, there are different hierarchical domains for considering the patterns of disruption of Sengupta et al. Editor's Page 925 Figure 1. Wave Front of the Ventricular Electrical Activation Sequence The activation sequence in the normal heart (Top) and with left bundle branch block (LBBB) conduction defect (Bottom) is presented; the anterior wall of the ventricle is flipped open and placed (cavity en face) on the right. The activation sequence is represented by the time scale color bar; the activation spreads in a wave front, with each colored zone representing successive 10 to 20 ms. During normal conduction, activation begins within both the left ventricular (LV) and right ventricular endocardium but in LBBB, activation begins only in the right ventricle and proceeds through the septum before reaching the LV endocardium. The line of activation sequence block in the anterior wall in LBBB is shown by a dashed line (between substantially different time zones). Figure developed on the basis of data from Durrer et al. (1) and Strauss et al. (11), by Craig Skaggs. cardiac activation and the potential benefits of CRT. We urge investigators to provide a better mechanistic understanding, such that the seemingly complex events in cardiac electrical, muscle, and flow mechanics observed during ventricular dyssynchrony are simplified. Identifying potential responders to CRT is an extremely important and vexing question in the present-day care of patients with heart failure and an important piece of the puzzle, which will be solved only by addressing the cascade of activation-contractionflow sequences. Address for correspondence: Dr. Jagat Narula, Icahn School of Medicine at Mount Sinai, Mount Sinai Heart, One Gustave L. Levy Place, Mail box 1030, New York, New York 10029. E-mail: [email protected]. 926 Sengupta et al. Editor's Page JACC: CARDIOVASCULAR IMAGING, VOL. 6, NO. 8, 2013 AUGUST 2013:924–6 REFERENCES 1. Durrer D, van Dam RT, Freud GE, Janse MJ, Meijler FL, Arzbaecher RC. Total excitation of the isolated human heart. Circulation 1970;41:899–912. 2. Ramanathan C, Jia P, Ghanem R, Ryu K, Rudy Y. Activation and repolarization of the normal human heart under complete physiological conditions. Proc Natl Acad Sci U S A 2006;103:6309–14. 3. Ashikaga H, Coppola BA, Hopenfeld B, Leifer ES, McVeigh ER, Omens JH. Transmural dispersion of myofiber mechanics: implications for electrical heterogeneity in vivo. J Am Coll Cardiol 2007;49:909–16. 4. Ashikaga H, van der Spoel TI, Coppola BA, Omens JH. Transmural myocardial mechanics during isovolumic contraction. J Am Coll Cardiol Img 2009;2:202–11. 5. Sengupta PP. Exploring left ventricular isovolumic shortening and stretch mechanics: “The heart has its reasons..” J Am Coll Cardiol Img 2009;2:212–5. 6. Zareba W, Klein H, Cygankiewicz I, et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 2011;123:1061–72. 7. Auricchio A, Fantoni C, Regoli F, et al. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation 2004;109:1133–9. 8. Sohal M, Shetty A, Duckett S, et al. Non-invasive assessment of left ventricular contraction patterns using cardiac magnetic resonance imaging to identify responders to cardiac resynchronization therapy. J Am Coll Cardiol Img 2013;6:864–73. 9. Sengupta PP, Pedrizzetti G, Kilner PJ, et al. Emerging trends in CV flow visualization. J Am Coll Cardiol Img 2012;5:305–16. 10. Goliasch G, Goscinska-Bis K, Caracciolo G, et al. CRT improves LV filling dynamics: insights from echocardiographic particle imaging velocimetry. J Am Coll Cardiol Img 2013; 6:704–13. 11. Strauss DG, Selvester RH, Lima JA, et al. ECG quantification of myocardial scar in cardiomyopathy patients with or without conduction defects: correlation with cardiac magnetic resonance and arrhythmogenesis. Circ Arrhythm Electrophysiol 2008;1: 327–36.