* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Dinesh-ohiostate05
Survey
Document related concepts
Transcript
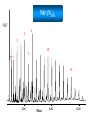
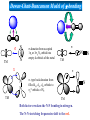
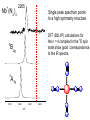
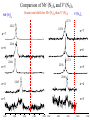
Infrared Photodissociation Spectroscopy of TM+(N2)n (TM=V,Nb) Clusters E. D. Pillai, T. D. Jaeger, M. A. Duncan Department of Chemistry, University of Georgia Athens, GA 30602-2556 www.arches.uga.edu/~maduncan/ U.S. Department of Energy Why Study TM-Nitrogen? • Biological systems require N2 as components of proteins, nucleic acids, etc. But N2 is highly inert (IP = 15.08 eV, BE = 225 kcal/mol). Nitrogenases catalyze N2 reduction and carry metal centers such as Fe, Mo, V. • Large scale ammonia synthesis uses Fe as catalyst. N2 + H2 Fe catalyst 350 - 1000 atm 2NH3 300 - 500 oC • N2 is isoelectronic to CO, C2H2 which are prevalent throughout inorganic and organometallic chemistry • N2 activation gauged by change in N-N bond distance or N-N vibrational frequency Previous Work • Electronic spectroscopy of M+(N2) (M = Mg, Ca) by Duncan and coworkers. • CID studies by Armentrout and coworkers for Fe and Ni with N2 • FT-ICR studies by H.Schwarz and coworkers, and electronic spectroscopy by Brucat and coworkers on Co+(N2) • Theoretical studies on TM-N2 carried out by Bauschlicher • ESR spectra for V(N2)6 and Nb(N2)6 done by Weltner. • IR studies using matrix isolation on M(N2) (M = V, Cr, Mn, Nb, Ta, Re) done by Andrews and coworkers Experimental Bond Energies* Direct absorption in our experiments is not possible due low ion densities. Solution is photodissociation. Ni+(N2)n IR photon 2359 cm-1 ~ 7 kcal/mol Small clusters may fragment via multiphoton process. Large clusters will be easier to fragment Fe+(N2)n n= 1 2 3 4 5 Bond Energy (kcal/mol) 13 19 10 13 15 n= 1 2 3 4 V+(CO)n n= 1 2 3 4 5 6 * Armentrout and coworkers Bond Energy (kcal/mol) 27 27 14 2 Bond Energy (kcal/mol) 27 22 17 21 22 24 LaserVision OPO/OPA 2000-4500 cm-1 Production of cold metal ion complexes with laser vaporization/ supersonic expansion. Mass selection of cations by time-of-flight. Tunable infrared laser photodissociation spectroscopy. Nb+(N2)n Nb+ 6 4 2 10 5 n= 1 16 200 Mass 400 600 Fragmentation of Nb+(N2)n 6 7 9 6 8 6 5 Fragmentation ends at n = 6 suggesting that this cluster is more stable. 7 n=6 100 200 300 mass 400 500 Infrared Photodissociation Spectra for Nb+(N2)n Free N2 mode 2359 cm-1 n=4 2265 Fragmentation is inefficient for the n = 1-3 clusters. n=3 The n=4 cluster shows fragmentation 95 cm-1 red of the free N2 stretch n=2 2100 2200 2300 cm -1 2400 Dewar-Chatt-Duncanson Model of p-bonding s N N TM s-donation from occupied 1pu or 3sg N2 orbital into empty d-orbitals of the metal s N TM p p N N TM N p- type back donation from filled dxy, dyz, dxz orbitals to pg* orbitals of N2 N TM Both factors weaken the N-N bonding in nitrogen. The N-N stretching frequencies shift to the red. N 2212 n=7 Spectra show a red shift of 95 cm-1 for n=4 as compared to free N2 stretch 2214 n=6 An additional red shift of 60 cm-1 is observed for n>4 cluster sizes 2204 n=5 n=4 The spectra of n=6 has a lower S/N ratio suggesting the complex is harder to dissociate owing to unusual stability 2265 n=3 2100 2200 2300 cm -1 2400 B3LYP/ DGDZVP Nb+ 6-311+G* N De= 33.8 kcal/mol Freq = 2291 cm-1 Osc. Strength = 55 km/mol De= 18.6 kcal/mol Freq = 2160 cm-1 Osc. Strength = 169 km/mol De= 8.3 kcal/mol Freq = 2209 cm-1 Osc. Strength = 376 km/mol De= 19.7 kcal/mol Freq = 2262 cm-1 Osc. Strength = 354 km/mol 1. DFT calculations favor linear over T-shaped structures ( De ~ 15 – 20 kcal/mol 2. T-shaped complexes red-shift N-N stretch by 150-200 cm-1 whereas linear complexes red shift by 50-100 cm-1. Nb+ + Grnd state: 4d4 5D Nb (N2)3 1st state: 4d35s 5F 6.7 kcal/mol 2nd state: 4d4 3P 15.9 kcal/mol 5 A1 3 B2 2100 2200 2300 2400 Spectrum has two modes because there are only two equivalent N2 + Nb (N2)4 5 2265 Single peak spectrum points to a high symmetry structure. DFT (B3LYP) calculations for the n = 4 complex for the 5D spin state show good correspondence to the IR spectra. B2g 3 A1g 2100 2200 2300 cm -1 2400 What is causing the additional red shifts for the n>4 clusters ? Nb+(N2)5 1. 3 A2 5 In addition all spectra are single peak signifying that no isomers are present. B1 2. 2100 2200 Other structures such as T-shaped or inserted complexes? DFT studies consistently predict linear structures over T-shaped structures. Energy differences ~ 15 kcal/mol and 20 kcal/mol. 2300 2400 A change in spin state? DFT (B3LYP) calculations for the n = 5 for triplet spin state shows better correspondence to IR spectrum than the quintet state. Also triplet state is found to be lower in energy by ~ 15 kcal/mol Comparison of Nb+(N2)n and V+(N2)n Nb+(N Greater red-shifts for Nb+(N2)n than V+(N2)n 2)n V+(N2)n 2271 2212 2258 n=7 n=7 2214 n=6 n=6 2271 2204 2258 n=5 n=5 2288 n=4 2265 n=4 n=3 2100 n=3 2200 2300 2400 2500 2100 2200 2300 2400 2500 s N N TM p N N TM 1. N2 and CO are p-accepting ligands and so dp-back donation is expected to dominate the bonding interaction. 2. d orbitals more diffuse for second row TM leading to better s-d hybridization. 3. Frequency shifts for V+(N2)n and Nb+(N2)n seems to justify this reasoning. Conclusions IR spectroscopy coupled with DFT calculations of Nb+(N2)n reveals the structures of these clusters. The spectra show that N2 binds in an “end on” configuration to Nb+. The results also reveal possible evidence for a change in multiplicity in the metal cation due to solvation effects. The N-N stretch in Nb+(N2)n red shifts further than in V+(N2)n consistent with the previous conclusions based on various TM(CO)n systems that p-back donation is the more significant interaction in these TM-ligand systems. + Nb (N2)n n=10 n=9 n=8 2212 n=7 2214 n=6 2100 2200 2300 2400