* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Border zone geometry increases wall stress after - AJP
Survey
Document related concepts
Transcript
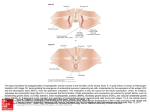
Am J Physiol Heart Circ Physiol 284: H475–H479, 2003; 10.1152/ajpheart.00360.2002. Border zone geometry increases wall stress after myocardial infarction: contrast echocardiographic assessment BENJAMIN M. JACKSON,1 JOSEPH H. GORMAN III,1 IVAN S. SALGO,3 SINA L. MOAINIE,1 THEODORE PLAPPERT,2 MARTIN ST. JOHN-SUTTON,2 L. HENRY EDMUNDS, JR.,1 AND ROBERT C. GORMAN1 1 Department of Surgery and the Harrison Department of Surgical Research and 2 Division of Cardiology, Department of Medicine, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania 19104; and 3Philips Medical Systems, Andover, Massachusetts 01810 Jackson, Benjamin M., Joseph H. Gorman III, Ivan S. Salgo, Sina L. Moainie, Theodore Plappert, Martin St. John-Sutton, L. Henry Edmunds, Jr., and Robert C. Gorman. Border zone geometry increases wall stress after myocardial infarction: contrast echocardiographic assessment. Am J Physiol Heart Circ Physiol 284: H475–H479, 2003;;10.1152/ajpheart.00360.2002.—After myocardial infarction (MI), the border zone expands chronically, causing ventricular dilatation and congestive heart failure (CHF). In an ovine model (n ⫽ 4) of anteroapical MI that results in CHF, contrast echocardiography was used to image shortaxis left ventricular (LV) cross sections and identify border zone myocardium before and after coronary artery ligation. In the border zone at end systole, the LV endocardial curvature (K) decreased from 0.86 ⫾ 0.33 cm⫺1 at baseline to 0.35 ⫾ 0.19 cm⫺1 at 1 h (P ⬍ 0.05), corresponding to a mean decrease of 55%. Also in the border zone, the wall thickness (h) decreased from 1.14 ⫾ 0.26 cm at baseline to 1.01 ⫾ 0.25 cm at 1 h (P ⬍ 0.05), corresponding to a mean decrease of 11%. By Laplace’s law, wall stress is inversely proportional to the product K 䡠 h. Therefore, a 55% decrease in K results in a 122% increase in circumferential stress; a 11% decrease in h results in a 12% increase in circumferential stress. These findings indicate that after MI, geometric changes cause increased dynamic wall stress, which likely contributes to border zone expansion and remodeling. congestive heart failure; remodeled myocardium; coronary artery disease; perfusion echocardiography (CAD) is the most common cause of congestive heart failure (CHF) in the United States (4). The mechanism of CHF due to CAD is complex and multifactorial. In addition to irreversibly damaged (infarcted) myocardium, the ischemic syndromes of myocardial stunning (2) and hibernating myocardium (13) contribute to the manifestations and progression of CHF in some patients with left ventricular (LV) dysfunction secondary to CAD. However, in many patients, myocardial infarction (MI) insidiously leads to CHF in the absence of ongoing ischemia or CORONARY ARTERY DISEASE Address for reprint requests and other correspondence: R. C. Gorman, Silverstein Pavilion, Hospital of the Univ. of Pennsylvania, 6th floor, 3400 Spruce St., Philadelphia, PA 19104 (E-mail: [email protected]). http://www.ajpheart.org recurrent infarction. Loss of contractile function in this later group is attributed to postinfarction LV remodeling (15). Remodeled myocardium has recently been defined clinically and experimentally as a unique form of dysfunctional myocardium adjacent to an infarct that is normally perfused but hypocontractile. The postinfarction remodeling process causes this region of hypocontractility to extend to involve progressively more normally perfused myocardium, resulting in ventricular dilatation and CHF (7, 12). The term border zone myocardium (BZM) has also been used in experimental and clinical studies to describe this phenomenon (3, 6, 10, 14) and is probably the better term when dealing with changes immediately after infarction. A recent experimental finite element stress analysis in an ovine model of fully developed LV aneurysm suggested that the mechanism underlying mechanical dysfunction in remodeled myocardium is primarily the result of an intrinsic abnormality of the myocardium rather than increased wall stress. The study, however, did not assess BZM stress in the early postinfarction period (5). The cause of these intrinsic myocardial changes has been difficult to determine given the complexity of the finite element stress calculations and the difficulty in identifying the area of remodeled myocardium over time. The latter requires serial registration of regional function and perfusion to adequately differentiate infarction from remodeled myocardium. In this study, we test the hypothesis that regional geometric changes consistent with increased BZM stress occur immediately after infarction. The term border zone will be used in preference to remodeled myocardium because of the time frame of the study (i.e., within 1 h of acute infarction). We employ an ovine model of anteroapical MI, a modified form of Laplace’s law, perfusion echocardiography (to precisely define BZM), and the assumption that the material properties of the BZM are unchanged immediately after infarction. The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked ‘‘advertisement’’ in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. 0363-6135/03 $5.00 Copyright © 2003 the American Physiological Society H475 Downloaded from http://ajpheart.physiology.org/ by 10.220.33.5 on May 13, 2017 Submitted 8 May 2002; accepted in final form 7 October 2002 H476 ALTERED BORDER ZONE GEOMETRY INCREASES WALL STRESS METHODS Fig. 1. Method of measuring endocardial radius of curvature (R). Left, baseline (without contrast); right, postinfarction. PPM, posterior papillary muscle; APM, anterior papillary muscle; RV, right ventricular cavity. point 1, septal border of the infarct; point 2, posterior border or observed flattening; point 3, midpoints of points 1 and 2. In all sheep, there was a sharp demarcation of the infarct at its septal border. The line of demarcation was decidedly distinct on cine echocardiogram; reference to the video allowed precise location of the infarct line (in yellow) in the still images. AJP-Heart Circ Physiol • VOL 284 • FEBRUARY 2003 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.5 on May 13, 2017 Surgical protocol. Four Dorsett hybrid sheep were induced with thiopental sodium (10–15 mg/kg), intubated, anesthetized with isofluorane (1.5–2%), and ventilated with oxygen (Drager anesthesia monitor, North American Drager; Telford, PA). All animals received glycopyrrolate (0.4 mg iv) and cefazolin (1 g iv both before and after operation). Animals were treated in compliance with National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, Revised 1985). The surface ECG and arterial blood pressure were continuously monitored (Sonometrics; London, Ontario, Canada). With the use of sterile surgical techniques, a left anterolateral thoracotomy was performed in the fifth interspace. Polypropylene snares were placed around the left anterior descending artery and second diagonal coronary arteries (⬃40% from the apex) (11). The animal was allowed to recover. Infarction. After 10–14 days, the sheep were again anesthetized and placed supine. An arterial catheter (model Spc350, Millar Instruments; Houston, TX) was positioned with its tip in the LV cavity. Baseline echocardiographic images were recorded as described below, without injection of contrast. The exteriorized subcutaneous snares were tightened. Once the animal stabilized (⬃1 h), experimental measurements were made. Echocardiographic data collection. Subdiaphragmatic echocardiographic images were obtained through a sterile midline laparotomy as previously described (8). Real-time contrast echocardiography was performed with a second harmonic technique (Sonos 5500, Hewlett-Packard). For each recording of perfusion and wall motion, 0.1 ml of albumen with octafluoropropane gas echo contrast agent (Optison, Mallinckrodt) was injected directly into the left coronary ostium. Short-axis cross-sectional images at the level of the papillary muscle bases were recorded continuously during the injection of contrast. Data analysis. In all sheep, there was a sharp demarcation of the infarct at its septal border (Fig. 1). The line of demarcation was decidedly distinct on cine echocardiogram; reference to the video allowed the precise location of the infarct line in the still images derived directly from the cine recording. The superiority of cine ultrasound for recognition and identification of cardiac structures and features is well recognized (1, 17). For acoustic reasons, the lateral border of the infarction was not as clearly demarcated with contrast; therefore, no measurements were performed at that border. All measurements were made at end systole, identified as the frame at which LV cavity area was smallest. The endocardial and epicardial contours were traced by an echocardiography technician who was unaware of the hypotheses of the study. In all sheep, the endocardial contour anterior to the infarct demarcation was observed to have flattened with infarction (in the short-axis plane). All measurements are presented as means ⫾ SD. Measurement of radius of curvature. As demonstrated in Fig. 1, three points were identified along the endocardial contour: the septal border of the infarct (point 1), the posterior border of the observed flattening (point 2), and their midpoint (point 3). After superposition of the baseline and postinfarction images such that both the posterior and anterior papillary muscles coincided, three analogous points were H477 ALTERED BORDER ZONE GEOMETRY INCREASES WALL STRESS a force. Because the wall stress in this case acts on all four sides of the square, the force opposing P is 4 䡠 p 䡠 Rd 䡠 h, where h is the thickness of the shell. Therefore P 䡠 R2 䡠 d2 ⫽ 4 䡠 䡠 d 䡠 Rd 䡠 h 2 And is ⫽ PR 2h RESULTS identified along the endocardial contour of each baseline echocardiographic study. For each contour, the three points allowed the calculation of the endocardial radius of curvature (R) and of its inverse (the curvature K). The coordinates (pixels) represented by points 1 and 2 were connected by a line segment and the bisector to this line segment was calculated; the same was done for points 2 and 3. The distance from the origins of the bisectors to their intersection was then taken to be R. Measurement of wall thickness. Myocardial wall thickness was determined in the border zone region by measuring, for each of the aforementioned three endocardial points, the distance to the closest point along the epicardial contour (again, at end systole). Border zone wall thickness was then calculated as the mean of these three measurements. Measurement of circumferential extent of endocardial flattening. The geometric center of the curve comprising the endocardial contour was taken to be the geometric center of the LV cavity. The angle subtended by points 1 and 2 around this center was measured to be the circumferential extent of the observed phenomenon. Mechanics model. Laplace’s law is applicable to differential areas of a three-dimensional shell under a pressure load. This can be demonstrated using a spherical balloon inflated to pressure P, as in Fig. 2. The force on a differential squareshaped piece of the surface of the sphere, as depicted, is P 䡠 R2 䡠 d2, where d is the differential sphere surface. As d approaches zero, the component of wall stress p in the direction of this force is p ⫽ 䡠 sin d d ⫽䡠 2 2 where is wall stress. Stress has the same units as pressure and must be multiplied by the area over which it acts to find AJP-Heart Circ Physiol • VOL Fig. 3. Endocardial contours (K) from a single animal at baseline (blue) and after infarction overlayed. The region of the infarct (red) can be seen to “billow,” as reflected in an increased K. As a result, the adjacent endocardium (green) necessarily demonstrates decreased K compared with baseline. 284 • FEBRUARY 2003 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.5 on May 13, 2017 Fig. 2. Laplace’s law as applied to a differential area of a threedimensional shell under a pressure load. , Wall stress; P, component of wall stress; A, surface area; A⬘, surface area upon which acts; d, differential sphere surface; P, pressure. In the border zone, K decreased from 0.86 ⫾ 0.33 cm⫺1 at baseline to 0.35 ⫾ 0.19 cm⫺1 at 1 h (P ⬍ 0.05), corresponding to a mean decrease of 55%. Note that the ventricles were all approximately the same size, with a mean radius of 2.4 ⫾ 0.3 cm. The area of decreased K extended 42° ⫾ 10° circumferentially from the infarct in the direction of preserved perfusion. Also in the border zone, h decreased from 1.14 ⫾ 0.26 cm at baseline to 1.01 ⫾ 0.25 cm at 1 h (P ⬍ 0.05), corresponding to a mean decrease of 11%. According to Laplace’s law, is inversely proportional to the product K 䡠 h in a differential segment of the LV wall. Therefore, a 55% decrease in K results in a 122% increase in circumferential . To demonstrate this, assume that c is a constant, that ⬘ is the new H478 ALTERED BORDER ZONE GEOMETRY INCREASES WALL STRESS circumferential stress, and that the new curvature K⬘ ⫽ 0.45 䡠 K. Then ⬘ ⫽ c c c ⫽ ⫽ 2.22 ⫽ 2.22 K⬘ 0.45 䡠 K K Similarly, a 11% decrease in h results in a 12% increase in circumferential . The final determinant of wall stress with the use of a Laplace’s law formulation is cavity pressure. In these experiments, the mean LV systolic pressure (LVP) did not change with infarction. At baseline, the LVP was 114 ⫾ 6 mmHg, and in the infarcted ventricles the LVP was 116 ⫾ 10 mmHg. DISCUSSION AJP-Heart Circ Physiol • VOL This work was supported by National Heart, Lung, and Blood Institute Grants HL-36308 and HL-63594, the Mary L. Smith Char- 284 • FEBRUARY 2003 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.5 on May 13, 2017 This study uses an ovine model of acute anteroapical MI that undergoes early expansion and real-time contrast echocardiography to study myocardial mechanics in BZM. According to the straightforward mechanics model (based on Laplace’s law for a differential segment of a thin-walled vessel) used in this study, the two determinants of wall stress are wall thickness and endocardial curvature. The results provide evidence that myocardial wall stress is increased in BZM immediately after MI and that this increase is primarily due to reduced endocardial curvature, with a more limited contribution from myocardial wall thinning. This study marks the first attempt to demonstrate altered border zone geometry after acute MI by using methods that allow definitive identification of BZM. Picard et al. (16) demonstrated the existence of a border zone 6 wk after MI in sheep, but evaluated wall motion abnormalities, not geometric changes. The present study demonstrates acute geometrical changes in the BZM adjacent to an expanding MI. Given the stable hemodynamic loading conditions before and after infarction, and by making the reasonable assumption that there is no significant acute change in local material properties during the first hour after MI, these geometric distortions are consistent with increased wall stress in this region of the left ventricle. These results suggest that in BZM the primary etiology of increased stress immediately after MI is early geometric change. These distortions in the border zone are likely due to the geometric boundary conditions imposed on the border zone by the adjacent infarct. The infarct expands and thins and is displaced radially outward from the center of the ventricle; to accommodate this distortion in the infarcted region, the adjacent myocardium is forced to curve outward, decreasing the local K, as indicated in Fig. 3. With the use of sonomicrometry array localization, serial microsphere injections, and this ovine model of anteroapical infarction, our laboratory has recently shown that an initially small area of normally perfused but hypocontractile BZM extends over weeks to involve progressively more normally perfused myocardium, resulting in ventricular dilatation and CHF (7). We have proposed that this enlarging area of dysfunctional but normally perfused myocardium is responsible for the CHF associated with postinfarction remodeling (7). Our findings confirm the results of Kramer and colleagues (9) demonstrating progressive BZM contractile dysfunction in this model up to 6 mo after infarction. Guccione and colleagues (5), using an elegant finiteelement stress analysis of this model, have suggested that the contractile dysfunction seen in the BZM 8 wk after infarction is due to intrinsic changes in the myocardium and not to increased stress. The results of our study suggest that, in contrast, early acute geometric changes in BZM lead to increased stress and as a result loss of contractility. We hypothesize that this early increase in stress causes the progressive and chronic intrinsic changes in BZM demonstrated by Guccione et al. (5). While remodeling progresses, these intrinsic changes in BZM become dominant to those related to the mechanical disadvantage associated with increased regional stress. The present study was limited in that two-dimensional echocardiograms were used to make conclusions about a three-dimensional ventricle. Two-dimensional endocardial curvature was measured; implications about myocardial wall stress were then inferred. Given the three-dimensional nature of the LV, three-dimensional contrast echocardiography would provide more comprehensive data and a more rigorous analysis. However, the two-dimensional (short axis) nature of this study is not necessarily a substantial limitation because the curvature in the orthogonal direction along the wall is less (the ventricle being longer in that direction), it is unlikely that a significant fraction of the pressure is opposed by stresses in the longitudinal (base-to-apex or long axis) direction. In addition, it is expected that a smaller fractional change in R is experienced in the long axis or meridional direction than in the short axis or hoop direction. The mechanics model utilized is of limited applicability: Laplace’s law is a first approximation for the determination of wall stress. In a thick-walled chamber with heterogeneous material properties, the approximation must be used with care and conclusions thereof must be considered preliminary. A more comprehensive model of myocardial mechanics, incorporating local endocardial curvature measured in two orthogonal directions (and incorporating three-dimensional data), a more rigorous theoretical treatment of thickwalled structures under load, and myocardial material properties, would clearly be more exact. However, the conclusions are still valid, given that 1) the acute change in local material properties in a normally perfused region of myocardium are likely minimal, and 2) the differential (in the radial direction) circumferential expansion of the border zone under the loads imposed during systole are likely not appreciably different than those endured by the normal myocardium in the uninfarcted ventricle. ALTERED BORDER ZONE GEOMETRY INCREASES WALL STRESS itable Trust (Chester, PA), and the W. W. Smith Charitable Trust (Chester, PA). REFERENCES AJP-Heart Circ Physiol • VOL 9. Kramer CM, Lima JA, Reichek N, Ferrari VA, Llaneras MR, Palmon LC, Yeh IT, Tallant B, and Axel L. Regional differences in function within noninfarcted myocardium during left ventricular remodeling. Circulation 88: 1279–88, 1993. 10. Lima JA, Becker LC, Melin JA, Lima S, Kallman CA, Weisfeldt ML, and Weiss JL. Impaired thickening of nonischemic myocardium during acute regional ischemia in the dog. Circulation 71: 1048–1059, 1985. 11. Markovitz LJ, Savage EB, Ratcliffe MB, Bavaria JE, Kreiner G, Iozzo RV, Hargrove WC III, Bogen DK, and Edmunds LH Jr. Large animal model of left ventricular aneurysm. Ann Thorac Surg 48: 838–845, 1989. 12. Narula J, Dawson MS, Singh BK, Amanullah A, Acio ER, Chaudhry FA, Arani RB, and Iskandrian AE. Noninvasive characterization of stunned, hibernating, remodeled and nonviable myocardium in ischemic cardiomyopathy. J Am Coll Cardiol 36: 1913–1919, 2000. 13. Rahimtoola SH. The hibernating myocardium. Am Heart J 117: 211–221, 1989. 14. St. John Sutton M, Pfeffer MA, Moye L, Plappert T, Rouleau JL, Lamas G, Rouleau J, Parker JO, Arnold MO, Sussex B, and Braunwald E. Cardiovascular death and left ventricular remodeling two years after myocardial infarction: baseline predictors and impact of long-term use of captopril: information from the Survival and Ventricular Enlargement (SAVE) trial. Circulation 96: 3294–3299, 1997. 15. Sakai K, Kozo W, and Millard RW. Defining the mechanical borderzone: a study in the pig heart. Am J Physiol Heart Circ Physiol 249: H88–H94, 1985. 16. Scherrer-Crosbie M, Liel-Cohen N, Otsuji Y, Guerrero JL, Sullivan S, Levine RA, and Picard MH. Myocardial perfusion and wall motion in infarction border zone: assessment by myocardial contrast echocardiography. J Am Soc Echocardiogr 13: 353–357, 2000. 17. Vuille C and Weyman AE. Left ventricle. I. general considerations, assessment of chamber size and function. In: Principles and Practice of Echocardiography (2nd ed.), edited by Weyman AE. Philadelphia, PA: Lea and Febiger, 1994. 284 • FEBRUARY 2003 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.5 on May 13, 2017 1. Amico AF, Lichtenberg GS, Reisner SA, Stone CK, Schwartz RG, and Meltzer RS. Superiority of visual versus computerized echocardiographic estimation of radionuclide left ventricular ejection fraction. Am Heart J 118: 1259–1165, 1989. 2. Braunwald E and Kloner RA. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation 66: 1146–1149, 1982. 3. Gallagher KP, Gerren RA, Stirling MC, Choy M, Dysko RC, McManimon SO, and Dunham WR. The distribution of functional impairment across the lateral border of acutely ischemic myocardium. Circ Res 58: 570–583, 1986. 4. Gheorghiade M and Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation 97: 282–289, 1998. 5. Guccione JM, Moonly SM, Moustakidis P, Costa KD, Moulton MJ, Ratcliffe MB, and Pasque MK. Mechanism underlying mechanical dysfunction in the borderzone of left ventricular aneurysm: a finite element model study. Ann Thorac Surg 71: 654–662, 2001. 6. Homans DC, Asinger R, Elsperger KJ, Erline D, Sublett E, Mikell F, and Bache RJ. Regional function and perfusion at the lateral border of ischemic myocardium. Circulation 71: 1038–1047, 1985. 7. Jackson BM, Gorman JH, Moainie SL, Guy TS, Narula N, Narula J, St. John Sutton MG, Edmunds LH, and Gorman RC. Extension of borderzone myocardium in postinfarction dilated cardiomyopathy. J Am Coll Cardiol 40: 1160–1167, 2002. 8. Kelley ST, Malekan R, Gorman JH III, Jackson BM, Gorman RC, Suzuki Y, Plappert T, Bogen DK, Sutton MG, and Edmunds LH Jr. Restraining infarct expansion preserves left ventricular geometry and function after acute anteroapical infarction. Circulation 99: 135–142, 1999. H479