* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemistry 2000 Lecture 20: Organic bases
Survey
Document related concepts
Nitrogen cycle wikipedia , lookup
Proteolysis wikipedia , lookup
Catalytic triad wikipedia , lookup
Point mutation wikipedia , lookup
Citric acid cycle wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Metalloprotein wikipedia , lookup
Peptide synthesis wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Butyric acid wikipedia , lookup
Genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Transcript
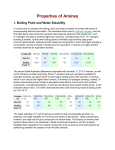
Chemistry 2000 Lecture 20: Organic bases Marc R. Roussel Organic bases I Other than the conjugate bases of organic acids we have already talked about, the only significant group of organic bases are compounds containing nitrogen atoms, mainly amines, although some others (e.g. imines, compounds that contain a carbon-nitrogen double bond) can also be reasonably strong bases. I Amines are analogs of ammonia, i.e. they are Lewis bases due to the lone pair on the nitrogen atom: R H N: R R H H R + N O: .. R R H − :O: .. Strength of bases I I As with acids, it’s convenient to have a quantitative measure of the strength of a base. Two measures are commonly used: 1. The pKa of the conjugate acid Weaker conjugate acid ⇒ stronger base Example: Acid NH+ CH3 NH+ 4 3 pKa 9.3 10.6 CH3 NH+ 3 is a weaker acid than ammonium, so methanamine (CH3 NH2 ) is a stronger base than ammonia. 2. Kb , the base ionization constant, is the equilibrium constant for the reaction of the base with water: B + H2 O BH+ + OH− pKb = − log10 Kb Larger Kb ⇒ smaller pKb ⇒ stronger base Strength of amines I As a rule, we find the following order: I ammonia primary amine tertiary amine least basic Two effects are competing here: secondary amine most basic 1. Increasing the number of alkyl substituents increases the opportunities for delocalizing the charge of the conjugate acid through an inductive effect: alkyl groups are more polarizable than hydrogen, so they are better at stabilizing the positive charge of the acid. 2. The acid form is stabilized by hydrogen bonding to water. Increasing the number of alkyl substituents decreases the number of hydrogen bonds that can be formed. I Example: Compound pKb NH3 4.79 CH3 CH2 NH2 3.37 (CH3 CH2 )2 NH 3.02 (CH3 CH2 )3 N 3.35 Inductive effects I Now consider the following pair of compounds: H + CH3 CH2 + I N CH2 C N Acid (CH3 CH2 )3 NH pKa 10.65 4.55 This large difference in pKa of the conjugate acids of these amines arises because of an inductive effect. CH2 CH3 I The highly electronegative nitrogen in the nitrile group withdraws electrons from its carbon atom, leaving the latter with a partial positive charge. I The proximity of this positive charge to the dissociable proton of the amine destabilizes this proton (by simple repulsion), making it more acidic. Amides I Delocalization of the lone pair makes amides extremely poor bases. Bases and pH I Given (p)Kb , we can calculate the pH of a solution containing the base. I One catch: We need aH+ to calculate pH, which we don’t get directly from a calculation involving Kb . I Use Kw = (aH+ )(aOH− ). Example: pH of a 0.045 M solution of ethanamine I For ethanamine (CH3 CH2 NH2 ), pKb = 3.37 at 25 ◦ C. I pH = 11.62 Kb and Ka I We have already mentioned that weaker bases have stronger conjugate acids and vice versa. I Consider the base ionization and acid dissociation equilibria for a base and its conjugate acid: B + H2 O BH+ + OH− BH+ B + H+ H2 O H+ + OH− (aBH+ )(aOH− ) aB (aB )(aH+ ) Ka = aBH+ Kw = (aH+ )(aOH− ) Kb = ∴ Kw = Ka Kb Amino acids in aqueous solution H 2N H O C C OH R I I I I Amino acids include both a carboxylic acid functional group and a basic amine functional group. Due to inductive effects, the two functional groups in an amino acid have slightly different pKa s than is typical for these functional groups. Carboxylic acids typically have pKa s of between 3 and 5. In an amino acid, the pKa is around 2. The pKa of an alkyl ammonium ion is usually between 10 and 11. In most amino acids, the pKa of the conjugate acid of the amine is between 9 and 10. I Exercise: Sketch the distribution curves for a typical amino acid. I Physiological pH is about 7, so what is the state of an amino acid in vivo? I In vivo, we expect to get a zwitterion: + H 3N H O C C O − R (Zwitter is a German adjective meaning “hybrid”.)