* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Melting and Magma Generation
Survey
Document related concepts
Transcript
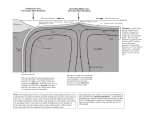
Why the Earth Melts EAS 458 Volcanology Lecture 7 How to Melt a Rock? Raise the temperature Heat production: radioactive heating, friction, impact heating Heat conduction or advection May occur in the crust, crust, particularly where high-T magma intrudes low solidus country rock, otherwise, conduction is not efficient Decompression If the adiabatic gradient and the solidus intersect, rising rock will melt. This is the most important melting mechanism. Therefore, in a certain sense, volcanoes are like clouds. Lower the melting point Just as salt lowers the freezing point of water, addition of some substances ( a flux) to rock can lower its melting point. Water is the most effective and most likely flux This is very likely important in subduction zones 1 Volcanism occurs at: Divergent plate boundaries Convergent plate boundaries More rarely in plate interiors Decompression Melting Decompression of rising mantle accounts for most volcanism on Earth, in particular, volcanism at divergent plate boundaries and in intraplate settings. With the help of thermodynamics, we can readily understand why this melting occurs. 2 Fundamental Variables T: temperature always absolute temperature, or Kelvins, Kelvins, in thermodynamics P: pressure force per unit area; SI unit is the Pascal (P) = 1 Newton/m2 = 1 kg-m/s2; in geology, we use MPa (106 Pa) or GPa (109 Pa). 1 atm ≈ 1 bar = 0.1 MPa. In the mantle pressure increases at a rate of ~ 1 GPa for each 35 km depth. V: volume Fundamental Variables U: Energy SI unit is the Joule Q: Heat: a form of energy W: Work: a form of energy dW = -PdV S: Entropy - strictly defined as the ratio of heat exchanged to temperature in a reversible process: S = ∆Q/T Also: S = k ln Ω where Ω is the number of states available to the system. a measure of the randomness of a system Units of J/K (Joules per Kelvin) 3 Derived Variables H: enthalpy: enthalpy: heat content, units of Joules ∆H is the energy absorbed (or given up) by a system as a consequence of heating, chemical reaction, or phase change ∆Hfus is the latent heat of fusion (heat required to transform a substance from solid to liquid at constant temperature). ∆Sfus = ∆Hfus/T CP: Heat capacity at constant pressure Relation to H and T: Cp = (∂ (∂ H/ H/∂ T) T)P α: coefficient of thermal expansion (fractional expansion of a substance upon heating) κ: thermal diffusivity: the heat flux is the product of κ times the thermal gradient. Derived Variables (A: Helmholz Free Energy: Energy: energy available for work) G: Gibbs Free Energy - energy available for nonPV work (e.g., chemical work) ∆G = ∆H - T∆ T∆S dG = VdP-SdT Two important properties: Products are reactants are at equilibrium when their Gibb Free Energies are equal. Chemical reactions always proceed in the direction if lower Gibbs Free Energy 4 Laws First Law of Thermodynamics: Conservation of Energy Equivalence of Heat and Work dU = dQ - dW Second Law of Thermodynamics The entropy of the universe always increases dS ≥ dQ/T Definitions System: That part of the universe under consideration Closed system: one which does not exchange mass with its surroundings Isolated system: one which exchanges neither mass nor energy with its surroundings. Adiabatic system: system: on which exchanges neither heat or mass with its surroundings, but which may do work or have work done on it. An adiabatic system is one for which dQ = 0; therefore, since dSrev = dQ/T, dQ/T, an adiabatic system is also isoentropic in the reversible case. 5 Definitions Solidus: temperature at which a substance (solution) begins to melt Liquidus: temperature at which a substance (solution) completely melts Convection and the Adiabatic Gradient We now know that the mantle convects (hot parts rise, cool parts sink) and that this is closely related to volcanism. Hot mantle rises beneath mid-ocean ridges and also mantle plumes. Because of the scales involved and the low thermal diffusivity of rock (10-6 m2-s-1), the rising rock can be viewed as approximately adiabatic. adiabatic. Question: How will the temperature of the rock change as it rises? (This is the same as asking what is the adiabatic gradient). Thermodynamically, we want to know (∂ (∂ T/ T/∂ P) P)S 6 Derivation of the Adiabatic Gradient “Adiabatic” Adiabatic” implies dS ≈ 0. Express the entropy change as a ‘generic’ generic’ function of T and P dS = (∂ (∂ S/ S/∂ P) P)TdP + (∂ (∂ S/ S/∂ T) T)PdT Since dS = 0 0 = (∂ (∂ S/ S/ ∂ P) P)T+ (∂ (∂ S/ S/∂ T) T)P (∂ T/ T/∂ P) P)S Rearranging: (∂ T/ / ∂ P) ) = (∂ ∂ S/ T P S ( S/∂ P) P)T/(∂ /(∂ S/ S/∂ T) T)P It can be shown that (∂ S/ S/∂ T) T)P = CP/T and (∂ S/ S/∂ P) P)T = -αV Thus: (∂ T/ T/∂ P) P)S = αVT/CP Adiabatic gradients depends on properties of the material (α (α, CP, V) and temperature. Clapeyron Equation Consider, for simplicity, a simple one-component system that melts completely at a single temperature. At the melting point (and only at that temperature), the solid and liquid phases are in equilibrium. Equilibrium implies dG = 0. Recall that dG = VdP-SdT For a reaction or phase change, such as melting, this equation may be written as d∆G = ∆VdP - ∆SdT where ∆ designates the change in the property upon reaction. Hence: ∆VdP - ∆SdT= 0; ∆VdP = ∆SdT 7 Clapeyron Equation From ∆VdP = ∆SdT we can derive: dT/dP = ∆V/∆ V/∆S This is known as the Clapeyron Equation and describes the slope of a phase boundary. Since ∆V and ∆S are functions of T and P, the phase boundary will generally be curved. For all substances, ∆Sfus is positive; for most substances ∆Vfus is positive. Hence the Clapeyron slope will generally be positive. In other words, the melting temperature will increase with increasing pressure. Decompression Melting Again, the adiabatic gradient is: (∂ T/ T/∂ P) P)S = αVT/CP Rising parcels of mantle will follow this T-P path. For olivine V ≈ 43.8 cc/mol (=J/MPa-mol); Cp ≈ 193 J/K-mol (at 1650 K), α = 2.7 x 10-5 K-1. At 1650 K, the adiabatic gradient is ~10K/GPa Solidus slope is: dT/dP = ∆V/∆ V/∆S ∆V ≈ 0.434 J/MPa/g; ∆S ≈ 0.362 J/K/g Slope of the solidus is ~120 K/GPa Solidus slope is much greater than adiabat, so rising mantle can cross it when it is hot enough. 8 Effect of Lithosphere Thick Lithosphere Thin Lithosphere 9 Melt Productivity Once the solidus is reached, how much melting occurs? We can again use thermodynamics to answer this question (at least approximately). Recall the partial differential for S: dS = (∂ (∂ S/ S/∂ P) P)TdP + (∂ (∂ S/ S/∂ T) T)PdT and (∂ S/ S/∂ T) T)P = CP/T and (∂ S/ S/∂ P) P)T = -αV We ask, how will entropy of one phase (e.g., the solid) change with pressure at the solidus? Thus: dSs/dP = CPs/T (∂ T/ T/∂ P) P)2φ -αsVs Melt Productivity Let F be the fraction of melt. The entropy of the whole system, S0, is: S0 = FSl + (1-F)Ss Rearranging: F = (S0 - Ss)/(S )/(Sl-Ss) = (S0 - Ss)/∆ )/∆Sfus. Differentiating with respect to pressure: (∂ F/ F/∂ P) P)S = 1/∆ 1/∆Sfus(CPs/T( /T(dT/dP) dT/dP)2φ - αsVs) 10 Melt Productivity Melt productivity is: (∂ F/ F/∂ P) P)S = 1/∆ 1/∆Sfus(CPS/T(dT/dP /T(dT/dP))2φ - αSVS) Assuming (dT/dP (dT/dP))2φ = ∆Vfus/∆Sfus Evaluating this for cpx at 1650 K: CP = 1.61 J/K/g ∆Sfus = 0.253 J/K/g ∆Sfus = 0.253 J/K/g Vs = 0.392 J/Mpa/g α = 3 x 10-5/K (∂ F/ F/∂ P) P)S = 6%/GPa ≈ 6%/35 km Will increase as with decreasing P and T Melt Productivity A little more math and we can derive the following: T )V " !T % *$ # !P '& F CP " !F % $# '& ( T !P S " !T % +Sm + $ # !F '& P Cp This is a more general relationship, and the term (∂ T/ T/∂ F) F)P is just the inverse of isobaric melt productivity and can be determined from experiments. 11 Melting in Subduction Zones In contrast to divergent plate boundaries, convergent plate boundaries or subduction zones are regions where sinking occurs. So it is hard to see how decompression melting can operate. While decompression may contribute to melting in subduction zones, flux melting may be more important. Water & Plate Tectonics Interaction of seawater and hot rock at mid-ocean ridges results in metamorphic reactions and formation of hydrous minerals such as chlorite, amphibole, and serpentine. Water content of “mature oceanic crust” crust” may reach several percent. Hydrous mineral such as these are stable only at relatively low temperatures. At high temperature, the reactions reverse and minerals break down to form dry minerals + water. 12 Effect of Water on the Solidus Island Arc Magmatism 13 Water Content & Melting in the Marianas Mantle flow in Subduction Zones 14 Summary Decompression melting accounts for most volcanism Rising mantle will intersect the solidus and melt, provided it is hot enough (generally is) and the lithosphere is thin enough (generally isn’ isn’t). Initial temperature and lithospheric thickness dictate the extent of melting. Accounts for volcanism at MOR’ MOR’s and mantle plumes “Flux melting” melting” (addition of water) is important in subduction zone melting. Melting by increasing T is rare (impact, crustal melting). 15