* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The effect of interruption of the short posterior ciliary arteries
Survey
Document related concepts
Transcript
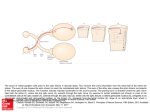
Reports 495 Volume 15 Number 6 dissection of eyes allowing regional flows to be compartmentalized more accurately in the present study. In addition, precautions were taken in the present study to maintain blood-gases and pH in the normal range, since variations in these can independently alter ocular blood flow.7 The discrepancy may be due to the use of the pig in the present study in contrast to previous studies,2' •'> n suggesting a species specific nature of the ocular vascular response to catecholamines. Finally, there is the possibility that vasodilation induced by isoproterenol is secondary to stimulation of ocular metabolic rate rather than vasodilation due directly to stimulation of beta-adrenergic receptors.1 Studies using specific antagonists of isoproterenol are needed to confirm the existence of beta-adrenergic receptors in the ocular vasculature. From the Departments of Physiology and Ophthalmology, Albany Medical College, Albany, N. Y. 12208. Supported in part by Grant 1R02 E7000791 from the National Eye Institute. Submitted for publication Sept. 12, 1975. Reprint requests: Dr. A. B. Malik, Department of Physiology, Albany Medical College, Albany, N. Y. 12208. Key words: microspheres, reference sample method, pigs, regional ocular flows, isoproterenol, norepinephrine. REFERENCES 1. Aim, A.: Effects of norepinephrine, angiotension, dihydroergotamine, papaverine, isoproterenol, nicotinic acid, and xanthinol nicotinate on retinal oxygen tension in cats, Acta Ophthalmol. 50: 707, 1972. 2. Chandra, S. R., and Freidmen, E.: Choroidal blood flow. II. The effects of autonomic agents, Arch. Ophthalmol. 87: 67, 1972. 3. Macri, F. T.: Local ganglion-line stimulating properties of some adrenergic amines which affect blood vessels of the anterior segment of the eye, INVEST. OPHTHALMOL. 13: 389, 1974. 4. Best, M., Gerstein, D., Wald, N. et al.: Autoregulation of ocular blood flow, Arch. Ophthalmol. 89: 143, 1973. 5. Dalske, F. H.: Pharmacologica reactivity of isolated 6. 7. 8. 9. ciliary arteries, INVEST. OPHTHAL- MOL. 13:389, 1974. Cole, D. F., and Rumble, R.: Effects of catecholamines on circulation in the rabbit iris, Exp. Eye Res. 9: 219, 1970. Bill, A.: Blood circulation and fluid dynamics in the eye, Physiol. Rev. 55: 383, 1975. Buckberg, G. D., Luck, J. C , Payne, B. D., et al.: Some sources of error in measuring regional blood flow with radioactive microspheres, J. Appl. Physiol. 31: 598, 1971. Prince, J. H., Diesem, D., Eglitis, I., et al.: Anatomy and Histology of the Eye and Orbit in Domestic Animals. Springfield, 111., 1960, Charles C Thomas, Publisher. Downloaded From: http://iovs.arvojournals.org/ on 05/13/2017 10. Hayreh, S. S.: The choriocapillaries, Albrecht Graefes. Arch. Klin. Ophthalmol. 192: 165, 1974. The effect of interruption of the short posterior ciliary arteries on slow axoplasmic transport and histology within the optic nerve of the rhesus monkey. NORMAN S. LEVY. Tritiated leucine was injected into the vitreous of rhesus monkey eyes to make it available for protein synthesis by the ganglion cells. The short posterior ciliary arteries were cut three hours later or several weeks prior to the leucine injection. A reduction of labeled protein within the retrolaminar optic nerve was seen in all eyes so treated. Autoradiography revealed a diffuse reduction of axoplasmic transport into these optic nerve heads. There was consistent evidence of focal obstruction of labeled protein at the interface between the lamina scleralis and retrolaminar optic nerve. Vacuoles appeared in the most severely affected areas. These histologic changes were followed by gliosis in the areas of ischemic damage. Glaucomatous cupping of the optic nerve head was not seen within six weeks following the induced ischemia. Recent studies suggest that the obstruction in axoplasmic transport seen following intraocular pressure elevation in both rhesus and owl monkeys is most likely ischemic in origin.1 -3 Ischemia of the optic nerve head can be produced without intraocular pressure elevation by compromise of the posterior ciliary arterial supply.4- 5 Under these conditions, the quantity of slow axoplasmic transport into the optic nerve is also reduced.0 The purpose of this study is to characterize the effects on slow axoplasmic transport of sugical interruption of the arterial blood supply to the nerve head over periods of varying duration. Methods and materials. These studies were performed in twelve optic nerves of six rhesus monkeys (Macaca midatta), weighing 3 to 4 kilograms. Each animal had an ocular examination including tonometry, biomicroscopy, and funduscopy. Photographic documentation of the fundus was obtained before and during the study. Electroretinography and visually evoked responses were obtained in some animals as previously described.6 The posterior ciliary arteries were exposed by lateral orbitotomy under ketamine and barbiturate anesthesia. The area at which the optic nerve leaves the eye was observed under the microscope and the short posterior ciliary vessels surrounding the optic nerve cut at that location. In the opposite eye of each animal, a similar ex- Investigative Ophthalmology June 1976 496 Reports B Fig. 1. The retinas from the two eyes of animal No. 47 are compared autoradiographically, after staining with Masson-trichrome (xl60). The retinas appear normal in both eyes. The retina of the treated eye (A) shows a reduction in grain density relative to the control at four days following interruption of the short posterior ciliary arteries. posure was obtained but the vessels were not severed. At periods varying from three hours prior to surgery to six weeks after the time of surgery, bilateral anterior chamber paracenteses were performed to remove the aqueous. Each animal then received from 0.15 to 0.25 ml. of leucine-3H (1 mCi. per milliliter) intraviheally into each eye. Four days later the monkeys were deeply anesthetized and the eyes, with ample optic nerve, enucleated and placed directly in formaldehyde. The optic nerve was excised just behind the globe and the eye callotted horizontally. The eyes were then washed and dehydrated in ascending concentrations of alcohol, followed by xylene. Xylene was replaced with paraffin, and the tissue was vacuum infiltrated for an hour, maintaining orientation. The resultant block was trimmed, and eight micra sections were serially cut. In the control eye, the matched tissue was processed identically. The retrolaminar optic nerve was cut into 1 mm. serial sections. These were digested in 1.0 normal sodium hydroxide at 60° C. for 24 hours. The superior half of the retina was digested in a Downloaded From: http://iovs.arvojournals.org/ on 05/13/2017 similar manner. Protein determinations were made. Appropriate standards of bovine albumin were treated as were the experimental samples. These samples were measured by standard techniques using a spectronic 20 colorimeter at 500 nm, Samples of this same tissue were counted in a Beckman LS-100 liquid scintillation counter in a cocktail of naphthalene, dioxane, and liquifluor in polyethylene vials. Three 10-minute counts on each sample were averaged and the background subtracted. All results exceeded background levels by at least a factor of five. Paraffin sections eight micra thick were placed on acid-cleaned, gelatin-chrome, alum-dipped slides and heated for 24 hours at 60° C. These slides were then washed serially in xylene, alcohol, and water. The slides were dipped in Ilford K-5, size B emulsion and placed in light-tight boxes at 4° C. for 60 days. They were developed in Kodak D-19 for two minutes at 21° C. and stained with Masson trichrome. Matched autoradiographic sections of the optic nerve head from each eye were compared. The autoradiographs defined the distribution of the labeled material within the optic nerve head and retina at the time of sacrifice. Volume 15 Number 6 Reports 497 Table I. Scintillation counts from the optic nerves of 4 monkeys four days after severing the posterior ciliary arteries d.p.m./mg. optic nerve protein, normalized to equivalent retinal radioactivity P.C.A. severed optic nerve Control optic nerve Difference 1 mm. behind globe 2 mm. behind globe 3 mm. behind globe 4 mm. behind globe 13,824 (± 9,484) 105,488 (± 25,874) 87% 3,174 (± 1,587) 79,788 (± 13,103) 96% 2,183 (± 618) 57,490 (± 11,494) 96% 6,919 (± 3,917) 36,137 (±4,787) Optic nerve sites at which axoplasmic transport was altered by severing the posterior ciliary arteries were determined and studied. Results. In eight eyes, from four animals, tritiated leucine was injected into the vitreous two to three hours prior to surgery. This should have permitted sufficient time for the synthesis of ganglion cell protein to be completed7 before the induction of alterations in blood dynamics. A four-day interval between the surgical procedure and the time of sacrifice was employed, so that slow axoplasmic transport to the optic nerve was well underway. Intraocular pressure fell to 6 to 8 mm. Hg in the treated eyes vs. 16 to 20 mm. Hg in the control eyes.5- ° Edema of the macula and optic nerve was apparent one week following surgery. Some pallor of the nerve head itself was present. The retinas of the treated eyes appeared similar in histologic appearance to the controls, except for areas of intraretinal and subretinal hemorrhage seen in two of the surgically treated eyes. There was a significant difference in the specific activity of the retinal labeled protein in each of the surgically treated eyes, ranging from 29 to 90 per cent less than the control. Autoradiographic reduction in the grain density was seen in the retina of the eye in which the short posterior ciliary arteries had been severed (Fig. 1). However, this reduction in specific retinal activity was small relative to the much larger reduction in the egress of labeled axoplasmic protein from the treated eyes. In Table I, the disintegrations per minute (d.p.m.) per milligram of optic nerve protein have been normalized to reflect equivalent retinal specific activity, so that only the differences in protein transport into the optic nerve are expressed. After this normalization, axoplasmic transport into the surgically treated nerves was at least 80 per cent less in every animal studied. This difference is consistent with the reduction noted in our previous study, three and six weeks after severing the short posterior ciliary arteries.(; Autoradiography of the optic nerve heads from the two eyes of each animal were compared (Fig. 2). The most prominent change was diffuse reduction in the grain density in the optic nerve heads of the treated eyes. At the posterior margin Downloaded From: http://iovs.arvojournals.org/ on 05/13/2017 of the lamina scleralis of the optic nerve head, labeled axoplasm appeared to be obstructed in the treated eyes only (Fig. 2). Vacuolization in the prelaminar optic nerve was noted in the most severely damaged nerve sections. After six weeks, the histologic picture at the optic nerve showed proliferation of glial tissue with replacement of some axonal bundles (Fig. 3). The point of maximum cup change six weeks following the procedure is seen. Although there is some enlargement of the cup, the picture contrasts markedly with glaucomatous cupping.3 Discussion. In this study, ischemic effects upon axoplasmic transport were separated from pressure effects by severing the short posterior ciliary arteries about the optic nerve head. The slow transport of axoplasmic protein into the optic nerve was then measured. The histology of the nerve head under these conditions was also studied. After normalizing to achieve equal retinal specific protein activity, ischemia produced in excess of 80 per cent decrease in the amount of labeled protein entering the retrolaminar optic nerve. There was a diminution in the density of grains seen autoradiographically in the prelaminar and laminar optic nerve head. In addition, focal abnormalities in transport at the level of the lamina scleralis were seen after severing the posterior ciliary vessels. Since the slow component of axoplasmic transport has a rate of 1.5 mm. per 24 hours,R only ganglion cell somata within 6 mm. of the optic nerve head were contributing to the observed transport four days after injection of the labeled precursor. Data from more distant cells were not measured in this study. The faster moving components of axoplasmic protein are known to be dependent upon focal high energy phosphate supplies at each point along the axon for continued transport. The same is believed true of the slow component. Prompt recovery of axoplasmic transport will occur after up to 1.5 hours of anoxia.0 Recovery of transport is delayed with anoxia up to four hours, and is irreversibly blocked after six hours of anoxia. These observations on fast axoplasmic transport may help to explain the reduction in transport observed in this study following the induction of ischemia. Investigative Ophthalmology June 1976 498 Reports B Fig. 2. This Masson-trichrome stained autoradiograph (xl60) with insets (x400) shows the optic nerve heads from both eyes of animal No. 47. At the posterior margin of the lamina scleralis in the eye with induced surgical ischemia of the short posterior ciliary arteries (A), labeled axoplasm appears to be obstructed (arrows). In the control (B) no such alterations in transport were noted at the lamina scleralis. Some histologic evidence of vacuolization and early gliosis is present in the treated optic nerve (A). There is also a reduction in grain density. The site of ischemic alteration in axoplasmic transport is identical to that seen with pressure elevation.3 The effect becomes most apparent at the terminus of the watershed of capillaries that supply the optic nerve head, namely at the posterior extent of the lamina scleralis. The focal obstruction of axoplasmic transport occurred at the posterior margin of the lamina scleralis, the terminus of the circulatory watershed. The extent of change was elsewhere diffuse both autoradiographically and histologically. Morphologically, the disc changes resembled acute ischemic optic neuropathy, with edema of the optic nerve and macula and some blurring of the disc margins. In longer duration studies, the neurons were histologically replaced with gliotic tissue. There was no evidence of glaucomatous cupping, either by funduscopy or histologically, but a slight saucerization was present due to the gliotic replacement of lost neurons. The phenomenon of cupping appears to require pressure elevation associated with ischemia. As vacuoles appear in the laminar and retrolaminar areas in the face of pressure elevation, the disc is capable of collapsing into the defect resulting from Downloaded From: http://iovs.arvojournals.org/ on 05/13/2017 the tissue loss. In our study the pressure was lowered, rather than raised, by the surgical interruption, so that there was no significant pressure gradient across the lamina scleralis. There was no cupping. Pressure, as well as laminar ischemia with vacuolization, appears necessary to effect the irreversible cupping process seen in chronic intraocular pressure elevation. A three-hour interval was used between injection of the tritiated leucine and the surgical interruption of blood flow, since the observations of Karlsson and Sjostrand7 in the rabbit had suggested that this time is sufficient for the completion of protein synthesis by the retina. It is apparent that more time is probably required in the rhesus monkey, since a decrease in specific activity of retinal labeled protein was observed in those eyes in which the short posterior ciliary arteries were interrupted. This observation also implies that a reduction in the blood flow required to sustain the retina occurred during the period of protein synthesis. Nutrition to the outer retina via the choriocapillaris was undoubtedly altered. Some flow destined for the retina was probably lost by shunting into the Reports 499 Volume 15 Number 6 2. Anderson, D. R., and Hendrickson, A.: Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve, INVEST. OPHTHALMOL. 13: 771, 1974. 3. Levy, N. S.: The effect of elevated intraocular pressure on slow axoplasmic transport in the rhesus monkey. Doctoral Dissertation. University of Chicago, 1975. 4. Armaly, M. F., and Araki, M.: Optic nerve circulation and ocular pressure: Contribution of central retinal artery and short posterior ciliary arteries and the effect on oxygen tension, INVEST. OPHTHALMOL. 14: 475, 1975. SKI Fig. 3. This section (xlOO) of the optic nerve head of animal No. 30 shows the maximal extent of histologic change and cupping observed six weeks after severing the short posterior ciliary arteries. There is glial proliferation and replacement of lost neurons. No retrodisplacement of the cup, nor glaucomatous cupping is present. A small amount of saucerization is noted. severed short ciliary arteries. In addition, spasm of the central retinal artery may have occurred as a result of the surgery. Histologic evidence of intraretinal hemorrhages in two eyes supports this. All probably contributed to the compromise in retinal nutrition during the period of protein synthesis. Our results contrast with those of Anderson and Davis10 who found no evidence of any histologic change in the optic nerve head of young squirrel monkeys following surgical interruption of the short posterior ciliary arteries. This may reflect their selection of the young squirrel monkey for study. Armaly and Araki4 have found a 79 per cent reduction in blood flow to the retrolaminar optic nerve of rhesus following ligation of the short posterior ciliary arteries. A decrease in blood flow of this magnitude would probably be sufficient over a period of time to explain the 80 per cent reduction in axoplasmic transport and the histologic changes observed. The technical assistance of Cheryl Curington is appreciatively acknowledged. From the Veterans Administration Hospital (MRIS 5221-02) and the Department of Ophthalmology, University of Florida, Gainesville, Fla. This study was supported in part by Fight for Sight, Inc. (G-507), New York, N. Y. Submitted for publication Nov. 3, 1975. Reprint requests: Dr. N. S. Levy, 4020 Newberry Rd., Gainesville, Fla. 32607. REFERENCES 1. Levy, N. S.: The effect of elevated intraocular pressure on slow axonal protein flow, INVEST. OPHTHALMOL. 13: 691, 1974. Downloaded From: http://iovs.arvojournals.org/ on 05/13/2017 5. Hayreh, S. S., and Baines, J. A.: Occlusion of the posterior ciliary artery. Ill, Br. J. Ophthalmol. 56: 754, 1972. 6. Levy, N. S., and Adams, C. K.: Slow axonal protein transport and visual function following retinal and optic nerve ischemia, INVEST. OPHTHALMOL. 14: 91, 1975. 7. Karlsson, J. C , and Sjostrand, J.: Synthesis, migration, and turnover of protein in retinal ganglion cells, J. Neurochem. 18: 749, 1971. 8. Williard, M., Cowan, W. M., and Bagelos, P. R.: The polypeptide composition of intraaxonally transported proteins. Evidence for four transport velocities, Proc. Natl. Acad. Sci. 71: 2183, 1974. 9. Leone, J., and Ochs, S.: Reversibility of fast axoplasmic transport following differing durations of anoxic block in vitro and in vivo. Program/Abstracts, Annual Meeting of the Society for Neuroscience, 3: 147, 1973. 10. Anderson, D. R., and Davis, E. B.: Retina and optic nerve after posterior ciliary artery occlusion: an experimental study in squirrel monkeys, Arch. Ophthalmol. 92: 422, 1974. Centrioles and cilia in the mesothelial cells of the pericanalicular region. M. GARY WlCKHAM AND DAVID M. WORTHEN. An evaluation of 70 trabecular meshwork biopsies obtained at the time of therapeutic surgery in glaucomatous and cataractous eyes revealed that the mesothelial cells in the iridocorneal angle had a marked abundance of cilia and centrioles. The distribution of cells showing cilia and/or centrioles is positively correlated with the apparent aqtieaus humor outflow pathway. The morphology and arrangement of the cilia-centriole complexes in the angle are highly variable and show many forms not previously reported in a single tissue. There were no obvious correlations between organelle abundance and the identifiable factors affecting the patients involved in this study. Scherft and Daems1 presented a review of the literature on the various types of cilia in vertebrates. Their data showed that most single or