* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Long-term Outcomes of Keratolimbal Allograft for Total Limbal Stem
Survey
Document related concepts
Transcript
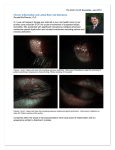
CLINICAL SCIENCES Long-term Outcomes of Keratolimbal Allograft for Total Limbal Stem Cell Deficiency Using Combined Immunosuppressive Agents and Correction of Ocular Surface Deficits Lingyi Liang, MD, PhD; Hosam Sheha, MD, PhD; Scheffer C. G. Tseng, MD, PhD Objective: To determine the long-term outcomes of kera- tolimbal allograft (KLAL). Methods: Scores of such risks as infrequent blinking, blink-related microtrauma, conjunctival inflammation, elevated intraocular pressure, dry eye, symblepharon, lagophthalmos, and previous KLAL or penetrating keratoplasty (PKP) failure were calculated and recorded before, during, and after KLAL. Prolonged oral mycophenolate mofetil and tacrolimus and short-term prednisone and acyclovir were administered in 12 eyes (10 consecutive patients) with total limbal stem cell deficiency after KLAL. Ten eyes underwent subsequent PKP. Results: More corrective measures were required in eyes with higher risk scores. During a follow-up of 61.2 months (standard deviation [SD], 18.2; range, 36-91 months) after KLAL, postoperative epithelial breakdown due to exposure occurred late in the period after PKP and re- T Author Affiliations: State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Ocular Surface Disease Center, Sun Yat-sen University, Guangzhou, China (Dr Liang); Ocular Surface Center, Ocular Surface Research and Education Foundation, Miami, Florida (Drs Liang, Sheha, and Tseng); and Research Institute of Ophthalmology, Cairo, Egypt (Dr Sheha). mained a primary risk. Mean daily doses of 1.4 g of mycophenolate mofetil and 1.6 mg of tacrolimus were administered for 52.7 months (SD, 22.5; range, 23-91 months) with few adverse effects and reached trough levels of 1.6 µg/mL (SD, 0.6 µg/mL) and 4.5 ng/mL (SD, 2 ng/mL), respectively. Keratolimbal allograft and PKP rejection was noted in 2 and 3 eyes, respectively, though there was a reversal in 1 eye in each group, yielding final KLAL and PKP survivals in 10 and 8 eyes, respectively, and ambulatory visual acuity of up to 20/20 in 10 eyes for 67.2% of the entire follow-up period. Conclusion: Correction of ocular surface deficits combined with an immunosuppressive regimen further improves the long-term outcome of KLAL in eyes with total limbal stem cell deficiency. Arch Ophthalmol. 2009;127(11):1428-1434 HE MAINTENANCE OF A healthy corneal epithelium relies on stem cells located at the limbus.1,2 When limbal stem cells or their supporting stromal environment is destroyed, corneal blindness occurs.3 Histologically, corneas with limbal stem cell deficiency (LSCD) carry the hallmark of conjunctivalization associated with chronic inflammation, superficial neovascularization, scarring, and poor epithelial integrity.4,5 Consequently, patients with LSCD experience severe photophobia and decreased vision. Conventional penetrating keratoplasty (PKP) is contraindicated. Visual rehabilitation for LSCD resorts to transplantation of autologous or allogeneic limbal stem cells, depending on the laterality, the extent of involvement, and the patient’s compliance.6 For bilateral total LSCD, or in unilateral total LSCD when the fellow eye is not chosen as a donor, transplantation of allogeneic limbal stem cells is achieved by either limbal (REPRINTED) ARCH OPHTHALMOL / VOL 127 (NO. 11), NOV 2009 1428 conjunctival allograft from living, related donors7,8 or keratolimbal allograft (KLAL) from cadaveric donors,7,9,10 or both.11 Compared with limbal conjunctival allograft from living, related donors,9,10,12,13 KLAL provides more stem cells without compromising the healthy limbal tissue in a normal eye. Transplantation of an allograft poses a high risk of rejection even in HLAmatched recipients. Therefore, KLAL survival depends on systemic immunosuppression for a prolonged, if not indefinite, period.6 Immunosuppression using topical and systemic steroids and cyclosporine A cannot halt allograft rejection in highrisk PKP14 or KLAL.13,15-17 Combined systemic immunosuppression based on mycophenolate mofetil and tacrolimus has been shown to be more effective and safer than cyclosporine A in promoting the survival in solid organ transplantations by lessening acute rejection18 and can reduce rejection in high-risk PKP.19 However, it remains unclear whether this regimen is also effective WWW.ARCHOPHTHALMOL.COM Downloaded from www.archophthalmol.com by VadrevuRaju, on March 12, 2010 ©2009 American Medical Association. All rights reserved. Table 1. Preoperative Clinical Characteristics and Risk Factors Preoperative Risk Factors and Scores, No. Case No./ Sex/ Age, y 1/M/41 2/M/46 3/F/59 4/M/70 5/M/65 6/M/28 7/F/64 8/M/50 9/M/27 10/M/52 Eye Cause of LSCD Previous Operations Left Idiopathic Right CB Left CB Right SJS LKP Right CB AMT; CE⫹IOL Left CB Right CB Left CB 2 PKPs, trabeculectomy Left CB CE⫹IOL, KLAL, AMT, T Right CB T Left CB KLAL, 2 PKPs, CE⫹IOL Left CB AMT, CE⫹IOL, 2 KLALs Previous Failure Infrequent Conjunctival Increased Dry Pathogenic Total Blinking BRMT Inflammation IOP Eye Lagophthalmos Symblepharon PKP KLAL Score 0 1 1 1 1 1 1 1 1 1 1 1 0 0 0 1 1 1 1 1 0 1 0 1 0 1 1 0 1 1 1 0 0 0 1 1 0 0 0 0 1 0 1 1 1 1 1 1 0 0 0 1 0 1 1 0 0 1 1 1 0 0 0 0 0 0 0 0 1 1 0 1 0 0 0 0 0 0 0 0 1 0 0 1 0 0 0 0 0 0 0 2 0 0 2 0 0 0 0 0 0 0 0 0 1 0 1 2 0 2 2 3 4 4 5 5 5 5 7 9 Abbreviations: AMT, amniotic membrane transplantation; BRMT, blink-related microtrauma, CB, chemical burn; CE, cataract extraction; FR, fornix reconstruction; IOL, intraocular lens implantation; IOP, intraocular pressure; KLAL, keratolimbal allograft; LKP, lamellar keratoplasty; LSCD, limbal stem cell deficiency; PKP, penetrating keratoplasty; SJS, Stevens-Johnson syndrome; T, tarsorrhaphy. in prolonging the survival of KLAL and, if so, what the optimal long-term dosage is. Although KLAL rejection is considered the major cause of failure,6 it only accounts for a part of reported cases.16,20 In fact, such risk factors as keratinization,21 symblepharon,16 inflammation,22 and dry eye23 have been implicated for KLAL failure. We wonder whether there are asyet-unknown clinical variables that might affect the KLAL outcome and whether measures taken to correct these risk factors may augment aforementioned immunosuppression to improve the KLAL outcome. METHODS PATIENTS This study was approved by the Baptist Health South Florida institutional review board (Miami, Florida) to retrospectively review the medical records of 10 patients (12 eyes) with LSCD who were consecutively operated on by 1 surgeon (S.C.G.T.) at the Ocular Surface Center, Miami, from 2001 through 2005. All patients completed at least 36 months of follow-up after KLAL. Their clinical data concerning preoperative diagnosis, previous operations, measures taken to correct ocular surface deficits, outcomes, and complications were retrieved. Diagnosis of LSCD was confirmed in 2 eyes by impression cytology that showed goblet cells on the corneal surface24,25 and in 10 eyes by late fluorescein staining, loss of limbal palisade of Vogt, and superficial vascularization. OCULAR SURFACE DEFICIT SCORES To gauge the overall risk that may lead to graft failure or rejection, we arbitrarily assigned a total score by assigning 1 point for each of the following 7 ocular surface deficits: infrequent blinking; blink-related microtrauma due to eyelid margin abnormalities, such as malposition, scar, keratinization, or misdirected lashes as previously defined26; conjunctival inflammation; increased intraocular pressure (IOP); aqueous tear–deficient dry eye detected by the fluorescein clearance test27; lagophthalmos; and pathogenic symblepharon as recently defined28; as well as 1 point each for prior KLAL or PKP failure (Table 1). SURGICAL TECHNIQUES OF KLAL AND AMNIOTIC MEMBRANE TRANSPLANTATION All operations were performed under general anesthesia and included KLAL and amniotic membrane transplantation, similar to what has previously been reported with some modifications. 1 7 Briefly, pannus and scar tissue were removed from entire limbal and corneal surfaces. The denuded corneal and limbal and some of the bulbar surface was covered by a sheet of cryopreserved amniotic membrane (Bio-Tissue, Miami) with sutures in 10 eyes and with fibrin glue (Baxter Inc, AG, Vienna, Austria) in 2 eyes. Following a 7.5- to 8.0-mm trephination, the remaining donor ring without HLA matching was prepared for KLAL by trimming off the excess sclera, removing the posterior half of the stroma, and tapering both corneal and scleral edges. After KLAL was secured to the recipient’s conjunctival edge with interrupted 10-0 nylon sutures, another layer of amniotic membrane was sutured by a purse-string running 10-0 nylon sutures in 11 eyes, or ProKera (Bio-Tissue) was inserted in 1 eye. Temporary sutured tarsorrhaphy was performed in 11 eyes and permanent tarsorrhaphy in 1 eye. IMMUNOSUPPRESSIVE REGIMENS All patients received both 1 g of oral mycophenolate mofetil and 1 mg of tacrolimus twice a day starting 1 week preoperatively and 0.5-mg/kg prednisone daily and 200 mg of acyclovir 5 times daily starting 3 days prior to surgery. Both prednisone and acyclovir were tapered off 2 to 3 months postoperatively when the ocular surface was not inflamed. The trough levels of mycophenolate mofetil and tacrolimus were checked every 1 to 3 months, and their doses were adjusted according to ocular surface inflammation and systemic adverse effects that mycophenolate mofetil and tacrolimus are known for based on blood pressure and levels of creatinine, blood urea nitrogen, complete blood count, platelets, and liver profile evaluated at baseline and every 1 to 3 months. When the ocular surface was not inflamed, dosages of mycophenolate mofetil and tacrolimus were tapered to 0.5 g and 0.25 mg/kg per day, respectively, and discontinued if irreversible graft failure or significant adverse effects were noted. (REPRINTED) ARCH OPHTHALMOL / VOL 127 (NO. 11), NOV 2009 1429 WWW.ARCHOPHTHALMOL.COM Downloaded from www.archophthalmol.com by VadrevuRaju, on March 12, 2010 ©2009 American Medical Association. All rights reserved. OTHER POSTOPERATIVE MANAGEMENT Postoperatively, topical ofloxacin (Allergan, Irvine, California) and prednisolone acetate, 1% (Allergan), or unpreserved dexamethasone, 0.1%, eye drops were applied 4 times a day. Treatment with ofloxacin was discontinued when epithelialization was completed but resumed if there was a corneal epithelial defect. Dexamethasone dosage was tapered off in 2 to 3 months after the ocular surface became uninflamed. The suture for temporary tarsorrhaphy was removed usually 2 weeks after surgery or when the amniotic membrane dissolved, and a bandage contact lens, such as Night and Day (CIBA Vision, Duluth, Georgia) and a scleral lens from Kontur Kontact Lens Co (Hercules, California) were inserted. During follow-up, increased limbal inflammation or vascular engorgement with or without corneal epithelial defects9 was treated with subconjunctival injection of 20 mg of triamcinolone (Bristol-Myers Squibb, Princeton, New Jersey) and hourly topical steroid drops and an increase in mycophenolate mofetil and tacrolimus dosages to the preoperative level. Any epithelial breakdown, such as irregular epithelium, superficial punctate keratopathy, or epithelial defect, was promptly treated according to the underlying cause. Infrequent blinking and/or increased scleral show was managed by encouraging blinking and by punctal occlusion with or without autologous serum 4 times a day, followed by permanent tarsorrhaphy. A bandage contact lens was used continuously and an autologous serum was used until an intact epithelium remained stable. For eyes with total punctal occlusion and extended wear of bandage contact lens, irrigation of preservative-free saline solution twice daily was instructed. Recurrent trichiasis was corrected by periodic epilation. Eyelid malposition or deformity, resulting in blink-related microtrauma and/or lagophthalmos, was corrected by eyelid reconstruction. Pathogenic symblepharon was treated by symblepharon lysis, fornix reconstruction, and amniotic membrane transplantation as recently reported.28 OUTCOME MEASURES The first outcome measure was the change in best-corrected visual acuity from before to after surgery and in the postoperative period during which ambulatory vision (defined as a best-corrected visual acuityⱖ20/200) was maintained similar to visual acuities reported by Dohlman and Terada29 in patients with keratoprosthesis. The second outcome measure was KLAL survival, evidenced by the maintenance of an intact and stable corneal epithelium postoperatively. In contrast, KLAL failure was indicated by recurrent late fluorescein staining, conjunctivalization of the cornea, or persistent epithelial breakdown after the aforementioned corrective measures.17 The third outcome measure was conventionally defined PKP survival, while PKP failure was diagnosed by classic signs of endothelial or epithelial rejection lines and subepithelial infiltrates, keratic precipitates, stromal edema, and corneal opacity. RESULTS These 10 patients included 8 males and 2 females with a mean age of 50.2 years (standard deviation [SD], 15 years). Limbal stem cell deficiency was bilateral in 8 and unilateral in 2 patients (cases 9 and 10) and caused by chemical burns (10 eyes [83%]) and Stevens-Johnson syndrome (case 3); case 1 was idiopathic. Table 1 lists cases/ eyes ranked by preoperative risk score. The more severe cases (cases 6 to 10) had received more prior operations. Except for case 1, all had infrequent blinking. Blinkrelated microtrauma, conjunctival inflammation, increased IOP, and aqueous tear–deficient dry eye were found in more than 50% of eyes. Lagophthalmos or pathogenic symblepharon was found in cases 7 to 10, and prior PKP or KLAL failure was found in cases 6 to 10. Preoperatively, measures were taken to correct all ocular surface deficits, except for infrequent blinking (Table 2). A nonpreservative steroid was applied in 7 eyes with conjunctival inflammation. Intraocular pressure was controlled medically in 4 eyes and by implantation of a glaucoma drainage device in another 3 eyes. Punctal occlusion, artificial tears, and/or autologous serum was applied in all 6 eyes with dry eye. Eyelid reconstruction was performed in 2 eyes with blink-related microtrauma caused by eyelid malposition. Fornix reconstruction and amniotic membrane transplantation were performed in 2 eyes with pathogenic symblepharon as recently reported.28 Bandage contact lenses were inserted in 2 eyes with large epithelial defects. Epilation was also performed in those with misdirected lashes. Keratolimbal allograft/amniotic membrane transplantation was uneventfully performed in all 12 eyes. Intraoperatively, mitomycin C was applied in 5 eyes with conjunctivalinflammation,andfornixreconstructionwasperformed in 1 eye with remaining pathogenic symblepharon (Table 2). Postoperatively, the remaining infrequent blinking in all patients (except for case 2) was treated by a bandage contact lens or scleral lens immediately after the suture for temporary tarsorrhaphy was removed or when the amniotic membrane bandage dissolved. For case 2, a bandage contact lens was inserted at the first postoperative visit, ie, 2 months after KLAL, when an epithelial defect was noted. Punctal occlusion and autologous serum drops were administered in 3 and 5 eyes, respectively (Table 2). For a mean period of 9.6 months (SD,9.2; range, 1-31 months) after KLAL when the eye was not inflamed, 10 eyes in 8 cases (except for cases 1 and 3) underwent a total of 13 PKPs. Despite all aforementioned measures, 9 of 12 eyes still experienced a total of 14 episodes of corneal epithelial breakdown, including epithelial defects (n=10), superficial punctate keratopathy (n=3), and irregular epithelium (n=1) during a mean follow-up period of 61.2 months (SD, 18.2; range, 36-91 months) after KLAL. Notably, 13 of 14 episodes happened after PKP; among them 11 developed 4 months to 4 years later. Except for 2 eyes with the highest risk scores (cases 9 and 10), epithelial breakdown was resolved by additional measures (n=7), including additional punctal occlusion and autologous serum drops—with or without permanent tarsorrhaphy in 5 eyes in which epithelial breakdown (cases 2-6) was caused by infrequent blinking with or without increased scleral show. One such example is shown in Figure 1A and B. The corneal epithelial defect (Figure 1C and D) in 1 eye (case 7) caused by lagophthalmos and pathogenic symblepharon healed after fornix reconstruction (Figure 1E and F). The epithelial defect (Figure 1G) in another eye (case 8) caused by lagophthalmos due to skin burn led to the first PKP failure (Figure 1H), though it was resolved after skin and oral mucosal grafts and permanent tarsorrhaphy. These measures resulted in a stable corneal epithelium 27 months after a repeat PKP (Figure 1I and J). A total of 7 (SD, 3; range, 2-12) corrective measures were required, with more used in eyes with high risk scores (Table 2). Specifically, 9 eyes with a risk score of more than 2 needed 6 or more corrective measures, while the remaining 3 eyes with risk scores of less than 3 needed 5 or fewer corrective measures. The 3 eyes (REPRINTED) ARCH OPHTHALMOL / VOL 127 (NO. 11), NOV 2009 1430 WWW.ARCHOPHTHALMOL.COM Downloaded from www.archophthalmol.com by VadrevuRaju, on March 12, 2010 ©2009 American Medical Association. All rights reserved. Table 2. Corrective Measures, Operations, Complications, and Final Outcome Corrective Measures Case No. Preoperative 1 2 3 4 5 6 7 8 9 10 Intraoperative T T PO, AS, epilation, LR AG, epilation, PO PO, AS, epilation PO, AS, AG, epilation Epilation, GDD GDD, FR, MMC, BCL PO, AG, epilation PO, AG, BCL PO, GDD, FR, MMC, epilation, LR Postoperative, Early/Late a Total No. PKP, mo PostKLAL 2 5 12 T BCL BCL, b PO, AS/#T BCL/#T 3 13 T BCL, AS 6 MMC, T BCL, AS/PO 7 5 MMC, T BCL, AS, PO 7 4 MMC, T 9 4 SPK (1.5 y PKP) T BCL, AS/PO, #T BCL/PO, AS/#T 7 3 #T BCL, PO/FR 8 18, 44 BCL/#T/#T/ LR ⫻ 2/LR 10 1,8 BCL/PO BCL, AS/#T 7 12 31 5, 39 SPK (6 mo PKP), ED (4 y PKP) ED (18 mo first PKP) ED (14 d first PKP), ED (1 mo second PKP), SPK (8 mo 2nd PKP), ED (2 y second PKP) ED (4 mo PKP) ED (11 mo second PKP) T, LR MMC, T MMC, T, FR Outcome Measures Complications Corneal Epithelial Breakdown (Time After KLAL/PKP) ED (2 mo KLAL), ED (13 mo PKP) Irregular epithelium (15 mo PKP) ED (17 mo PKP) Rejection KLAL PKP Follow-up, mo VA Before; After KLAL PKP 0 0 NA 0 36 78 20/300; 20/80 CF; 20/200 S S NA S 0 0 70 20/300; 20/20 S S 0 NA 84 20/200; 20/25 S NA 0 0 60 20/400; 20/40 S S 0 0 67 HM; 20/20 S S 1, R 0 67 HM; 20/20 S S 0 2, R 79 HM; 20/20 S S 0 0 50 CF; 20/60 S S 0 0 36 LP; 20/80 S S 0 2, R, IR 1, IR 1, IR 37 56 CF; HM LP; 20/400 F F F F Abbreviations: AG, anti-glaucoma medications; AS, autologous serum; BCL, bandage contact lens or scleral lens; CF, counting fingers; ED, epithelial defect; F, failure; FR, fornix reconstruction; GDD, glaucoma drainage device implantation; HM, hand motion; IR, irreversible; KLAL, keratolimbal allograft; LP, light perception; LR, eyelid reconstruction; MMC, mitomycin C; NA, not applicable; PO, punctual occlusion; PKP, penetration keratoplasty; R, reversible; S, successful; SPK, superficial punctuate keratopathy; T, temporary tarsorrhaphy; #T, permanent tarsorrhaphy; VA, visual acuity. a Measures taken immediately after KLAL (early) and later when corneal epithelial breakdown ensued (late). b Starting when ED occurs but not immediately after KLAL. without postoperative epithelial breakdown had a low risk score of less than 5; among them 2 did not undergo PKP. Systemic immunosuppression was continued in all 8 patients in whom it was successful without any known significant adverse effects, though it was discontinued in 2 patients in whom it had failed because of irreversible PKP rejection (case 9) and persistent hypertension (case 10). As a result, it had been used for 52.7 months (SD, 22.5 months) in all patients, ie, 86% of the entire follow-up period. Persistent hypertension and hyperbilirubinemia in case 10 were reversed after discontinuation of tacrolimus; transient gastric upset and loss of appetite in case 8 was spontaneously resolved. During the period without any rejection, the average daily medication doses were 1.5 g (range, 0.8-2 g) and 1.6 g (range, 1.2-2 g) of mycophenolate mofetil and tacrolimus, respectively, resulting in mean trough levels of 1.6 µg/mL (SD, 0.6 µg/mL; range, 0.8-2.7 µg/mL) and 4.5 ng/mL (SD, 2 ng/mL; range, 1.7-9.8 ng/mL), respectively. Among all 13 PKPs performed in 10 eyes, 3 were repeated because of a retrocorneal membrane (case 7), persistent corneal epithelial defect (case 8), and irreversible corneal graft rejection (case 10). As shown in Table 2, 2 eyes (17%) encountered a total of 3 episodes of KLAL graft rejection, which occurred 11, 36, and 48 months after KLAL; 3 eyes (30%) experiencedatotalof4episodesofPKPgraftrejection,which occurred 6, 11, 46, and 70 months after PKP. Rejections occurred in cases 5, 6, 9, and 10, which had risk scores of 5 or higher; 3 of them had more than 1 prior KLAL/PKP failure (Table 1 and Table 2). Three KLAL and PKP graft rejections occurred after discontinuation of mycophenolate mofetil and tacrolimus owing to noncompliance to or intolerance of the drugs (case 9 and 10). For the remaining 4 rejections, daily doses of 1.1 g (SD, 0.7 g) of mycophenolate mofetil (P=.3) and 1.8 mg (SD, 1.0 mg) of tacrolimus (P=.9) were taken, which is no different from the doses taken when there was no rejection. The trough level at the time of KLAL or PKP rejection was not available. Temporally, 5 of 7 episodes occurred shortly after at least 1 of the following ocular surface deficits: trichiasis, loose sutures, dry eye, and exposure because of lagophthalmos and exotropia. After the anti-rejection treatment and corrective measures, 2 of 3 KLAL rejections (Figure 2A and B) and 2 of 4 PKP rejections (Figure 2C) were successfully reversed. All irreversible rejections were noted in 2 eyes of cases 9 and10.Keratolimbalallograftgraftmeltatthe12-to3-o’clock position limbus was noted in 1 eye (case 10) with severe chemical burn after KLAL and coincided with the location of prior scleral ischemia (Figure 2D). This area subsequently developed partial LSCD, which was confirmed by late fluorescein staining (Figure 2E). However, this eye ended up with irreversible KLAL and PKP graft rejections (Figure 2F). Postoperatively, IOP increased in 6 eyes with prior ocular hypertension, but was controlled by anti-glaucoma medications. There was no postoperative infection. (REPRINTED) ARCH OPHTHALMOL / VOL 127 (NO. 11), NOV 2009 1431 WWW.ARCHOPHTHALMOL.COM Downloaded from www.archophthalmol.com by VadrevuRaju, on March 12, 2010 ©2009 American Medical Association. All rights reserved. A C E B A B C D E F D F G H I J Figure 1. Postoperative management of corneal epithelial breakdown. Poor contact lens fit and epithelial defect caused by infrequent blinking and increased scleral show in the right eye of case 2 (A) was corrected by punctal occlusion, autologous serum drops, and permanent tarsorrhaphy and remained stable for 63 months thereafter (B). Poor contact lens fit caused by recurrent symblepharon (C) and lagophthalmos (C, inset) in case 7 resulted in a corneal epithelial defect (D). After symblepharon lysis and amniotic membrane transplantation, a deep fornix (E), complete eyelid closure (E, inset), and a stable epithelium were maintained 14 months thereafter (F). Progressive cicatricial eyelid deformity (G) caused by lagophthalmos (G, inset) and persistent epithelial defect led to penetrating keratoplasty graft failure in case 8 (H). Eyelid margin reconstruction (I) allowed the eye to close completely (I, inset) and the cornea remained clear 27 months after the subsequent penetrating keratoplasty ( J). For visual rehabilitation, cataract extraction and intraocular lens implantation were performed in 1 eye after KLAL (case 3) and in 6 eyes during PKP. Bestcorrected visual acuity improved from hand movement to 20/200 (before KLAL) to ambulatory 20/200 to 20/20 in 10 eyes (cases 1 to 8) at the last visit. Furthermore, the ambulatory vision was maintained for 41 months (SD, 26.9; range, 3-90 months), accounting for 67.2% of the entire post-KLAL follow-up period. Ten of 12 eyes (83%) maintained uninflamed ocular surfaces, which is no dif- Figure 2. Postoperative complications. Perilimbal injection signifying early keratolimbal allograft (KLAL) rejection (A) was accompanied by several small epithelial defect spots (B), dry eye, and trichiasis (not shown) in the right eye of case 5. One week after subconjunctival injection of 20 mg of triamcinolone, an increase in the dose of mycophenolate mofetil from 500 mg to 1000 mg daily, a change to a soft bandage contact lens, and epilation, the eye became noninflamed without epithelial defect for 32 months (C). Keratolimbal allograft failure was associated with diffuse neovascularization and focal melt at the 12- to 3-o’clock position, where there was scleral ischemia (D, arrows) 1 year after KLAL in case 10. Vivid late fluorescein staining suggestive of limbal stem cell deficiency was noted 12 months later (E). This eye had irreversible KLAL and penetrating keratoplasty graft failure 3 months later (F). ferent from eyes receiving conjunctival limbal autograft (Figure 3). The second outcome measure showed that these eyes also maintained KLAL survival (Figure 3). The third outcome measure showed that 8 of 10 eyes (80%) maintained PKP survival, resulting in a stable and smooth corneal epithelium (Figure 3). The failure in cases 9 and 10, with the highest risk scores, was associated with irreversible KLAL and PKP rejection because of exposure refractory to all measures taken. COMMENT Although KLAL with or without PKP has been advised for restoring vision in eyes with total LSCD, its outcome is not simply determined by successful transplantation of limbal stem cells, but can also be adversely affected by several risk factors threatening ocular surface health.6 We know that corneal surface health depends on a stable preocular tear film that helps supply oxygen to an avascular clear cornea when the eye is open. To achieve this goal, ocular surface epithelia, all external adnexal glands, and eyelids work in concert via neuroanatomic integration by 2 neural reflexes controlling secretion of different tear components and eyelid blinking/closure.30 The present study illustrates what ocular surface deficits are prevalent and threatening and how corrective measures may augment the longterm success of KLAL for eyes with total LSCD. Among ocular surface deficits, we have identified infrequent blinking, blink-related microtrauma, conjunctival inflammation, increased IOP, aqueous tear–deficient dry eye, lagophthalmos, pathogenic symblepharon, and prior KLAL or PKP failure as potential risk factors. As summarized in Tables 1 and 2, 11 of 12 eyes initially had 1 or more risk factors before KLAL. Eyes with higher scores had more prior failed operations, required more corrective measures, developed more postoperative corneal epithelial breakdown, showed more KLAL/PKP rejections, resulted in irreversible rejection, and had overall poor outcomes. Keratinization21, symblepharon16, inflammation22, and dry eye23 have been (REPRINTED) ARCH OPHTHALMOL / VOL 127 (NO. 11), NOV 2009 1432 WWW.ARCHOPHTHALMOL.COM Downloaded from www.archophthalmol.com by VadrevuRaju, on March 12, 2010 ©2009 American Medical Association. All rights reserved. recognized as potential risk factors in eyes receiving KLAL. In this study, only case 3 had mild eyelid margin keratinization, which caused blink-related microtrauma. Glaucoma is a known risk factor for PKP.31 Schwartz et al32 proposed a prognostic staging system according to underlying diseases with or without inflammation. Besides these, we also identified 3 other risk factors, namely, infrequent blinking, blink-related microtrauma, and lagophthalmos; the former 2 were present in the overwhelming majority of our cases, while the last 1 was presented in 3 severe cases (Table 1). Together, these 3 factors could destabilize the preocular tear film, hence contributing to postoperative epithelial breakdown and PKP failure as illustrated in case 8 (Figure 1G and H). Blink-related microtrauma could further damage the ocular surface epithelium and cause inflammation.26 Future studies are needed to determine whether a risk score as proposed herein can help predict the outcome in studies with a larger sample size. Identification of these risk factors helped us formulate a corrective strategy before, during, and after KLAL. Nine of 12 eyes required at least 6 types of corrective measures (Table 2). Similar to what has been reported,15-17,23 punctalocclusion,artificialtears,autologousserum,bandagecontact lenses, and tarsorrhaphy were needed in all patients (except for case 1) (Table 2). Previously, epithelial defect was considered 1 major postoperative complication in 36% to 73% of eyes receiving KLAL with or without PKP,15-17,23 while 37%16 to 83%23 of these defects lead to PKP failure. We believed that such postoperative complications should also include superficial punctate keratopathy and any irregular epithelium, as they might precede an epithelial defect (cases 2, 5, 6, and 8). Previous studies, however, did not provide information linking epithelial breakdown to PKP. Herein, we noted that such epithelial breakdown still developed late after PKP in 9 of 12 eyes despite all aforementioned measures. A continuous follow-up to protect against any insult to the ocular surface cannot be overstated. Such conventional measures as punctal occlusion, artificial tears, and autologous serum drops were not sufficient. Extended bandage contact lens wear not only protected the eye from blink-related microtrauma during blinking, but also held a stable precorneal tear film in eyes with infrequent blink. We did not note any bandage contact lens–associated infectious keratitis during the entire follow-up, when eyes also received topical antibiotic eye drops only during epithelialization and daily irrigation with preservative-free saline. The remaining exposure problem due to increased scleral show was solved by permanent tarsorrhaphy, while problems due to lagophthalmos were solved by other reconstructive operations. As a result, 7 of 9 eyes with epithelial breakdown healed and maintained a stable corneal epithelium and helped improve the surgical outcome. Combined immunosuppressive agents also play a role in our long-term maintenance of a clear and functional graft. Before KLAL, patients had their routine check-ups with an internist to rule out underlying diseases, such as significant hypertension, liver or kidney dysfunction, and hematocytopenia, all of which may affect the use and dosage of systemic immunosuppressants. During immunosuppressant treatment, internists and transplant specialists were consulted if there was any issue related to toxicity. The goal of immunosuppression is to keep the eye uninflamed, which A B C D E F G H Figure 3. Preoperative and postoperative appearances of eyes with successful treatment. A vascularized and scarred cornea of case 3 following prior lamellar keratoplasty (A) became stable and clear with a best-corrected visual acuity (BCVA) that improved from 20/200 to 20/25 for 84 months after keratolimbal allograft (KLAL) (B). A vascularized and scarred cornea in case 4 (C) had BCVA improve from 20/400 to 20/40 and a clear and stable cornea 60 months after KLAL (D). A vascularized and scarred cornea in the left eye of case 5 (E) had BCVA recover from hand motions to 20/20 with a stable and clear cornea 67 months after KLAL (F). A vascularized and scarred cornea of case 6 (G) became clear and safe, with BCVA improving from hand motions to 20/20 seventy-nine months after KLAL (H). the ophthalmologist monitors to avoid systemic adverse effects, which are monitored by an internist. Our daily dosages were less than 2 g of mycophenolate mofetil and 2 to 12 mg of tacrolimus, which are routinely used in liver or renal transplantation, and reached an average trough level that was also less than the target level of 8 to 13 ng/mL for tacrolimus.33-35 This might explain partly why there were fewer and milder adverse effects in these patients than in those receiving liver or renal transplantation.33-35 Alloway et al36 also noted that combined mycophenolate mofetil and tacrolimus resulted in significantly fewer systemic adverse effects in KLAL patients than renal transplant patients. The PKP rejection rate was 30%, not significantly different from 46% (28 eyes),15 57% (7 eyes),23 and 64% (14 eyes)25 (all P⬎.05)whensystemiccyclosporineAwasusedalone.However, the KLAL rejection rate was 17%, which is significantly lower than the 80% rate in 5 eyes receiving a daily dose of 4.4 mg of tacrolimus alone37 and 87.5% in 15 eyes receiving cyclosporine A alone (both P⬍.01).16 Because 5 of 7 rejections occurred shortly after trichiasis, loose sutures, (REPRINTED) ARCH OPHTHALMOL / VOL 127 (NO. 11), NOV 2009 1433 WWW.ARCHOPHTHALMOL.COM Downloaded from www.archophthalmol.com by VadrevuRaju, on March 12, 2010 ©2009 American Medical Association. All rights reserved. dry eye, or remaining exposure, we believe that the primary cause of rejection was recurring or persistent ocular surface deficits. Indeed, 4 such episodes were reversed after adjusting immunosuppressant doses and correcting the remaining ocular surface deficits. It is likely that previous failed KLALs in eyes with the highest risk scores decreased the success rate of subsequent KLALs despite adnexal surgery, corrective measures, and strong immunosuppression. Future studies are needed to address this likelihood and to determine whether these eyes require a different immunosuppressive regimen. Judged by all 3 outcome measures, aforementioned corrective measures and combined immunosuppressive treatment resulted in a long-term (more than 3 years) success rate of 80% to 83%, significantly higher than the long-term (over 2 years) success rate of less than 50% reported by others (a total of 121 eyes) (P⬍.05) with various ocular surface diseases13,15-17,23 but comparable with the long-term (an average of 3 years) success rate of 74.2% in 31 eyes (P⬎.05) with aniridia11 when oral cyclosporin A alone is administered. Future studies with a larger sample size may help determine the relative importance between measures taken to correct different ocular surface deficits in various ocular surface diseases and the combined immunosuppressive regimen in attaining the long-term success of KLAL with or without PKP in eyes with total LSCD caused by different diseases. Submitted for Publication: January 10, 2009; final revision received March 24, 2009; accepted March 30, 2009. Correspondence: Scheffer C. G. Tseng, MD, PhD, Ocular Surface Center, 7000 SW 97 Ave, Ste 213, Miami, FL 33173 ([email protected]). Financial Disclosure: Dr Tseng and his family are more than 5% shareholders of TissueTech Inc, which owns US patents 6,152,142 and 6,326,019 on the method of preparation and clinical uses of cryopreserved human amniotic membrane and ProKera distributed by Bio-Tissue Inc. No other author has any proprietary interest in any material mentioned in this study. Funding/Support: Supported in part by grants 2006B36006002 from the Technological Project Foundation of Guangdong Province and B2006061 from the Medical Science Fund of Guangdong Province (Dr Liang). REFERENCES 1. Schermer A, Galvin S, Sun T-T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103(1):49-62. 2. Cotsarelis G, Cheng SZ, Dong G, Sun T-T, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57(2):201-209. 3. Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78(3):433-446. 4. Puangsricharern V, Tseng SCG. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102(10):1476-1485. 5. Dua HS, Saini JS, Azuara-Blanco A, Gupta P. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol. 2000;48(2):83-92. 6. Espana EM, Di Pascuale M, Grueterich M, Solomon A, Tseng SC. Keratolimbal allograft in corneal reconstruction. Eye. 2004;18(4):406-417. 7. Holland EJ, Schwartz GS. Epithelial stem-cell transplantation for severe ocular surface disease. N Engl J Med. 1999;340(22):1752-1753. 8. Daya SM, Ilari L. Living related conjunctival limbal allograft for the treatment of stem cell deficiency. Ophthalmology. 2001;108(1):126-133. 9. Tsai RJF, Tseng SCG. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994;13(5):389-400. 10. Tsubota K, Toda I, Saito H, Shinozaki N, Shimazaki J. Reconstruction of the corneal epithelium by limbal allograft transplantation for severe ocular surface disorders. Ophthalmology. 1995;102(10):1486-1496. 11. Holland EJ, Djalilian AR, Schwartz GS. Management of aniridic keratopathy with keratolimbal allograft: a limbal stem cell transplantation technique. Ophthalmology. 2003;110(1):125-130. 12. Tan DTH, Ficker LA, Buckley RJ. Limbal transplantation. Ophthalmology. 1996; 103(1):29-36. 13. Holland EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc. 1996;94:677-743. 14. Hill JC. Systemic cyclosporine in high-risk keratoplasty: short- versus longterm therapy. Ophthalmology. 1994;101(1):128-133. 15. Tsubota K, Satake Y, Kaido M, et al. Treatment of severe ocular surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340 (22):1697-1703. 16. Ilari L, Daya SM. Long-term outcomes of keratolimbal allograft for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109(7):1278-1284. 17. Solomon A, Ellies P, Anderson DF, et al. Long-term outcome of keratolimbal allograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology. 2002;109(6):1159-1166. 18. Boratyńska M, Banasik M, Patrzalek D, Klinger M. Conversion from cyclosporinebased immunosuppression to tacrolimus/mycophenolate mofetil in patients with refractory and ongoing acute renal allograft rejection. Ann Transplant. 2006; 11(2):51-56. 19. Tabbara KF. Pharmacologic strategies in the prevention and treatment of corneal transplant rejection. Int Ophthalmol. 2008;28(3):223-232. 20. Maruyama-Hosoi F, Shimazaki J, Shimmura S, Tsubota K. Changes observed in keratolimbal allograft. Cornea. 2006;25(4):377-382. 21. Holland EJ, Schwartz GS. The evolution of epithelial transplantation for severe ocular surface disease and a proposed classification system. Cornea. 1996; 15(6):549-556. 22. Samson CM, Nduaguba C, Baltatzis S, Foster CS. Limbal stem cell transplantation in chronic inflammatory eye disease. Ophthalmology. 2002;109(5):862-868. 23. Shimazaki J, Shimmura S, Fujishima H, Tsubota K. Association of preoperative tear function with surgical outcome in severe Stevens-Johnson syndrome. Ophthalmology. 2000;107(8):1518-1523. 24. Prabhasawat P, Tseng SCG. Impression cytology study of epithelial phenotype of ocular surface reconstructed by preserved human amniotic membrane. Arch Ophthalmol. 1997;115(11):1360-1367. 25. Tseng SCG, Prabhasawat P, Barton K, Gray T, Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116(4):431-441. 26. Cher I. Blink-related microtrauma: when the ocular surface harms itself. Clin Experiment Ophthalmol. 2003;31(3):183-190. 27. Pflugfelder SC, Tseng SCG, Sanabria O, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998;17(1):38-56. 28. Kheirkhah A, Blanco G, Casas V, Hayashida Y, Raju VK, Tseng SC. Surgical strategies for fornix reconstruction based on symblepharon severity. Am J Ophthalmol. 2008;146(2):266-275. 29. Dohlman CH, Terada H. Keratoprosthesis in pemphigoid and Stevens-Johnson syndrome. Adv Exp Med Biol. 1998;438:1021-1025. 30. Tseng SC, Tsubota K. Important concepts for treating ocular surface and tear disorders. Am J Ophthalmol. 1997;124(6):825-835. 31. Ayyala RS. Penetrating keratoplasty and glaucoma. Surv Ophthalmol. 2000;45(2): 91-105. 32. Schwartz GS, Gomez JAP, Holland EJ. Pre-operative staging of disease severity. In: Holland EJ, Mannis MJ, eds. Ocular Surface Disease Medical and Surgical Management. New York, NY: Springer; 2002:158-167. 33. Jorge S, Guerra J, Santana A, Mil-Homens C, Prata MM. Mycophenolate mofetil: ten years’ experience of a renal transplant unit. Transplant Proc. 2008;40(3):700-704. 34. Brum S, Nolasco F, Sousa J, et al. Leukopenia in kidney transplant patients with the association of valganciclovir and mycophenolate mofetil. Transplant Proc. 2008;40(3):752-754. 35. Müller H, Solari S, Zuniga C, et al. Immunosuppression with generic tacrolimus and mycophenolate mofetil in renal transplant recipients: preliminary report in Chile. Transplant Proc. 2008;40(3):705-707. 36. Alloway R, Mogilishetty G, Cole L, et al. Ocular surface transplant recipients experience minimal immunosuppression complications: implications for composite tissue transplants [abstract]. Am J Transplant. 2007;1058:419. 37. Sloper CM, Powell RJ, Dua HS. Tacrolimus (FK506) in the management of highrisk corneal and limbal grafts. Ophthalmology. 2001;108(10):1838-1844. (REPRINTED) ARCH OPHTHALMOL / VOL 127 (NO. 11), NOV 2009 1434 WWW.ARCHOPHTHALMOL.COM Downloaded from www.archophthalmol.com by VadrevuRaju, on March 12, 2010 ©2009 American Medical Association. All rights reserved.