* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CHIRON - ACS Division of Chemical Information
Pharmaceutical marketing wikipedia , lookup
Toxicodynamics wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Prescription costs wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug design wikipedia , lookup
Pharmacognosy wikipedia , lookup
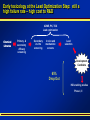
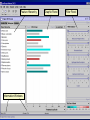
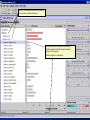
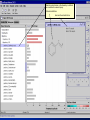
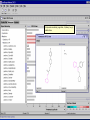
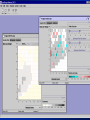
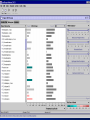
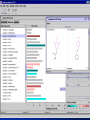
Computational Toxicology and Virtual Development in Drug Design Dale E. Johnson, Pharm.D., Ph.D. Chief Scientific Officer ddplatform LLC The “Problem” in pharmaceutical R&D • ~ $700 MM and over 10 years to develop novel drug • Approximately 75% of overall R&D cost attributed to failures The “Solution” for R&D • Identify/eliminate problematic drugs early • Design desirable properties into drugs Drug Discovery: the hunting process where is toxicology today? Target Selection Lead Identification Lead Optimization • Identification of potential targets • Screen development • Lead explosion/ optimization • Target verification • High-throughput screening • Potency in disease • Target selection • Secondary assays/ • Pharmacokinetics mechanism of action • Hits to leads From: Rosamond and Allsop, Science 287, 1973 (2000) • Early toxicology Early toxicology at the Lead Optimization Step: still a high failure rate – high cost to R&D ADME, PK, TOX Lead optimization Chemical Libraries Primary & secondary efficacy screening Secondary in vitro screening In vivo and mechanistic screens Lead selection Development Candidate 65% Drop Out IND enabling studies Phase I, II The toxicology solution • Incorporate predictive toxicology concept throughout discovery & development • Design reduced toxicity into chemical libraries • Create expert systems to accelerate and increase success rate – Expert systems must be multi-disciplinary for real impact Major needs in Predictive Toxicology: Recent industry surveys • Predictive software with updated databases • Improved data mining capabilities • Enhanced in vitro mechanistic screens • Ready access to human hepatocytes and other cells • Relevant application of new technologies ie. toxicogenomics Major needs in Predictive Toxicology: Recent industry surveys • Predictive software with updated databases • Improved data mining capabilities • Enhanced in vitro mechanistic screens • Ready access to human hepatocytes and other cells • Relevant application of new technologies ie. toxicogenomics Missing elements in the toolbox • Quality data from controlled sources • Newly created database(s) using “pharmaceutical” chemical space • Multi-disciplinary chem-tox Information / decision tools – Data mining via “med chem building blocks” • Flexibility to incorporate all data from internal and external sources • Web-based, platform independent LeadScopeTM Technology • Structural analysis based on familiar structural features • Powerful graphical representations and dynamic querying • Refine structure alerts to reflect new assay results • Statistically test structural hypotheses RTECS database & liver toxicity • ~7000 compounds with liver toxicity codes • Expert conversion to grades (risk) – Ordinal ranks using severity of findings, dose, regimen, species • Create 1o liver tox – chemical space • Data mining with ToxScopeTM: correlations between chemical structure and liver toxicity Feature Hierarchy Information Windows Graphic Panel Filter Panel Portion of the Heterocycles hierarchy showing 3 levels of the pyridine subhierarchy Selected subset of compounds containing a pyridine substructure with an acyclic alkenyl group in the 2-position Subset contains 2 compounds Each structure feature in the hierarchy is defined as a substructure search query Structural definition atom and bond restrictions Compounds containing a pyridine, 2-(alkenyl, acyc) substructure Uncovering bias in chemical space within data sets • Detect + and – coverage within a desired chemical space • Understand decision errors that can be introduced with biased space Structural alerts • Can rapidly find structural alerts • Can view new libraries in relation to structural alerts • Can evaluate impact of alert on optimization scheme RTECS grade 5 only ToxScopeTM Components • LeadScopeTM Enterprise Technology • Several public or commercial databases • New databases using “pharmaceutical" chemical space – New specific organ toxicity database * Structural alerts – Continual updates on target organs Conclusion “… an in silico revolution is emerging that will alter the conduct of early drug development in the future.” “Preclinical safety must transition from an experimental-based process into a knowledge-based, predictive process, where experimentation is used primarily to confirm existing knowledge” Acknowledgements Grushenka Wolfgang, Co-author Julie Roberts Bill Snyder Chris Freeman Don Swartz Ilya Utkin Wayne Johnson Allen Richon Paul Blower Glenn Myatt Emily Johnson Kevin Cross Michael Crump Jeff Miller Michael Murray Mark Balbes Zhicheng Li Yan Wang Limin Yu Sighle Brackman Lisa Balbes