* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Intracardiac Echocardiography-Guided Interventions
Survey
Document related concepts
Transcript
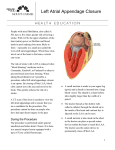
JACC: CARDIOVASCULAR INTERVENTIONS VOL. 7, NO. 9, 2014 ª 2014 BY THE AMERICAN COLLEGE OF CARDIOLOGY FOUNDATION PUBLISHED BY ELSEVIER INC. ISSN 1936-8798/$36.00 http://dx.doi.org/10.1016/j.jcin.2014.07.001 EDITORIAL COMMENT Intracardiac Echocardiography-Guided Interventions Do We Need Trials To Prove Equivalency/Superiority to Transesophageal Echocardiography?* Ziyad M. Hijazi, MD, MPH,yz Hussam Suradi, MDy I t may be surprising to realize that the initial work Pre-procedural echocardiography is required to screen in intracardiac echocardiography (ICE) imaging suitable candidates and to define LAA morphology appeared in the early 1960s, preceding the devel- and dimension. Periprocedural echocardiography has opment of interventional catheterization procedures a major role in guiding and deployment of the device as (1). The increasingly sophisticated catheter-based well as for screening for complications and assessment structural interventions that have emerged over the of procedural success. Post-procedural echocardiog- past decade, stimulated the growth of ICE to become raphy is important in the surveillance and monitoring an established imaging modality that holds distinct of long-term outcome, including complications. TEE is advantages for use in practice such as transseptal widely considered the gold standard tool in visualizing access (2), atrial and ventricular septal defects closure the LAA and is typically used to guide implantation of (3), and radiofrequency ablation for cardiac arrhyth- the LAA device. To obviate the need for endotracheal mias (4). Furthermore, in our center, we use it and esophageal intubation, there has been increased exclusively to guide all congenital and structural interest in performing the procedure under ICE guid- interventional procedures. As opposed to transeso- ance only. Over the past decade, there have been phageal echocardiogram (TEE), ICE offers compara- several reports discussing the utilization of ICE in ble, if not superior, imaging without the need for guiding LAA closure; however, to date, comparative general anesthesia. The use of ICE also allows the studies between the 2 imaging modalities in guiding interventionalist to simultaneously perform the pro- LAA closure are lacking (6,7). cedure as well as the imaging part without the need In this issue of JACC: Cardiovascular Interventions, Berti et al. (8) contribute significantly to the current of additional echocardiographic personnel. atrial experience regarding the utility of periprocedural ICE appendage (LAA) closure into clinical practice has as an alternative to TEE in guiding LAA occlusion stirred considerable interest to reduce the risk of procedures. This is the largest reported observational thromboembolism in patients with atrial fibrillation, study from 2 centers addressing the application of with several clinical trials demonstrating high proce- ICE as an imaging modality in guiding LAA device The introduction of percutaneous left dural success rates and noninferiority to warfarin for preventing embolic stroke (5). The procedure is fairly SEE PAGE 1036 intricate with echocardiographic guidance being closure. They report the short-term and mid-term re- an essential tool at all stages of this procedure. sults from 121 patients with mean age of 77 years, who underwent ICE-guided percutaneous LAA occlusion using Amplatzer Cardiac Plug I and II devices (St. Jude *Editorials published in the JACC: Cardiovascular Interventions reflect the Medical, St. Paul, Minnesota). All patients had non- views of the authors and do not necessarily represent the views of JACC: rheumatic atrial fibrillation with high stroke risk and Cardiovascular Interventions or the American College of Cardiology. absolute contraindication for oral anticoagulation. The From the ySidra Cardiovascular Center of Excellence, Sidra Medical and ICE catheter was positioned either in the right atrium Research Center, Doha-Qatar, Qatar; and the zRush Center for Congenital and Structural Heart Disease, Rush University Medical Center, Chicago, or coronary sinus. From those positions, the LAA Illinois. Both authors have reported that they have no relationships dimension was defined and followed by implantation relevant to the contents of this paper to disclose. of the appropriate device size. The LAA dimension 1046 Hijazi and Suradi JACC: CARDIOVASCULAR INTERVENTIONS VOL. 7, NO. 9, 2014 SEPTEMBER 2014:1045–7 Intracardiac Echocardiography-Guided Interventions obtained by ICE correlated well with those obtained navigating the ICE catheter within the heart and to by angiography and, to a lesser degree, with pre- obtain pristine images. Although not explicitly stated procedural TEE. In the majority of cases, the initial in the study methodology, it is assumed that all of the device size selected based on ICE measurements was operators in the study were highly skilled in ICE implanted successfully. Excellent technical (96.7%) imaging. Another unavoidable criticism is that, and procedural outcomes (93.4%) were achieved with the investigators report a high level of agreement 4 patients having major adverse events. These data regarding device size selection based on ICE and pre- support the pre-existing literature reporting the procedural TEE imaging. However, the validity of feasibility of ICE imaging in guiding LAA device closure such a conclusion is compromised as the operators as an acceptable alternative to TEE (9). However, what were not blinded to the 2 imaging modalities. More- conclusions can be drawn regarding the broad appli- over, we agree with the investigators that it may be cability using ICE to assist in such interventions? inappropriate to generalize the results of the study to Although we do believe that ICE imaging is able to all ICE systems, because a wide variety of ICE systems perform the intraprocedural tasks typically provided is commercially available with variable transducer by TEE, nevertheless, as the investigators highlight in functionality. Finally, patients left with relevant their discussion, there are several deficiencies in the residual peridevice leakages experience no real benefit study that may limit its broad applicability. Because from the procedure and have to stay on anti- accurate echocardiographic measurement of the LAA coagulation therapy. The investigators outline in their anatomy is critically important for successful device discussion the importance of residual peridevice leak; closure, using ICE instead of TEE to routinely guide however, they do not comment on either the echo- LAA device implantation would only be clinically cardiographic or angiographic assessment of device practical if ICE imaging were able to produce high- sealing of the LAA, which is an important variable of quality images consistently. Although we recognize the success of the procedure. that there is no generally accepted standard protocol The investigators should be praised for their excel- for LAA imaging with ICE, it is self-evident that com- lent technical and procedural outcomes with results plete imaging of this complex 3-dimensional structure very comparable to those reported with TEE-guided requires multiple 2-dimensional planes at a minimum implantation (11). Although imaging with ICE is inva- to properly define its dimensions. In this study, LAA sive and may increase costs, it offers less procedural imaging was obtained by positioning the ICE catheter discomfort for the patient and potentially reduces the either in the right atrium or coronary sinus; however, procedural time. It also eliminates the need for an the investigators offer no consistent methodology anesthesiologist and echocardiographic personnel, (views) by which the imaging was performed or enhancing in turn the cost-effectiveness of using this description of the quality of the images acquired. In imaging tool. Although it can be concluded from this our experience, right atrial views are rarely sufficient study that the use of periprocedural ICE is feasible and to visualize the complex anatomy of the LAA, partly safe in guiding LAA device closure, this study, like most due to the variable interatrial septum orientation and observational studies, serves as an initial step toward a distance from the LAA to the ICE position in the right pivotal trial that will compare the 2 imaging modalities atrium (the LAA lies in the echo far field, which often in relation to LAA device closure. Perhaps future en- requires lowering the frequency to increase the imag- deavors should be directed toward eliminating the ing field depth, resulting in significantly compromised need for pre-procedural TEE such that all the tasks far-field tissue resolution). This imaging limitation can required for LAA device closure can be achieved solely be easily overcome by placing the ICE catheter in the by ICE imaging thereby minimizing the excessive cost left pulmonary artery, in closer proximity to the LAA. and risk associated with duplicative TEE studies. Similarly, positioning the catheter in the left pulmonary artery offers superior image quality of the LAA REPRINT REQUESTS AND CORRESPONDENCE: Dr. and ease of probe manipulation as opposed to that in Ziyad M. Hijazi, Sidra Cardiovascular Center of the coronary sinus (10). Certainly, there is a significant Excellence, P.O. Box 26999, Doha-Qatar, Qatar. learning curve in order to gain proficiency in E-mail: [email protected]. REFERENCES 1. Cieszynski T. [Intracardiac method for the investigation of structure of the heart with the aid of ultrasonics]. Arch Immunol Ther Exp (Warsz) 1960;8:551–7. 2. Mitchel JF, Gillam LD, Sanzobrino BW, Hirst JA, McKay RG. Intracardiac ultrasound imaging during transseptal catheterization. Chest 1995; 108:104–8. 3. Hijazi Z, Wang Z, Cao Q, Koenig P, Waight D, Lang R. Transcatheter closure of atrial septal defects and patent foramen ovale under intracardiac echocardiographic guidance: feasibility and Hijazi and Suradi JACC: CARDIOVASCULAR INTERVENTIONS VOL. 7, NO. 9, 2014 SEPTEMBER 2014:1045–7 comparison with transesophageal echocardiography. Catheter Cardiovasc Interv 2001;52: 194–9. 4. Chu E, Kalman JM, Kwasman MA, et al. Intracardiac echocardiography during radiofrequency catheter ablation of cardiac arrhythmias in humans. J Am Coll Cardiol 1994;24: 1351–7. Intracardiac Echocardiography-Guided Interventions 6. Meier B, Palacios I, Windecker S, et al. Transcatheter left atrial appendage occlusion with Amplatzer devices to obviate anticoagulation in patients with atrial fibrillation. Catheter Cardiovasc Interv 2003;60:417–22. 7. MacDonald ST, Newton JD, Ormerod OJ. Intracardiac echocardiography off piste? Closure of the left atrial appendage using ICE and local anes- 5. Reddy VY, Doshi SK, Sievert H, et al., for the PROTECT AF Investigators. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in thesia. Catheter Cardiovasc Interv 2011;77:124–7. Patients with Atrial Fibrillation) trial. Circulation 2013;127:720–9. 9. Ho IC, Neuzil P, Mraz T, et al. Use of intracardiac echocardiography to guide implantation of a left 8. Berti S, Paradossi U, Meucci F, et al. Periprocedural intracardiac echocardiography for left atrial appendage closure: a dual-center experience. J Am Coll Cardiol Intv 2014;7:1036–44. atrial appendage occlusion device (PLAATO). Heart Rhythm 2007;4:567–71. 10. Reddy VY, Neuzil P, Ruskin JN. Intracardiac echocardiographic imaging of the left atrial appendage. Heart Rhythm 2005;2:1272–3. 11. Park JW, Bethencourt A, Sievert H, et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv 2011;77: 700–6. KEY WORDS atrial fibrillation, imaging-guided interventions, intracardiac echocardiogram, left atrial appendage occlusion, transeophageal echocardiogram 1047