* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Trematodes (Flukes)
Myxobolus cerebralis wikipedia , lookup
Toxocariasis wikipedia , lookup
Dirofilaria immitis wikipedia , lookup
Sarcocystis wikipedia , lookup
Fascioloides magna wikipedia , lookup
Echinococcosis wikipedia , lookup
Trichinosis wikipedia , lookup
Onchocerciasis wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Fasciola hepatica wikipedia , lookup
Nanophyetus salmincola wikipedia , lookup
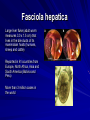
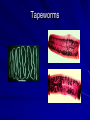
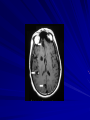
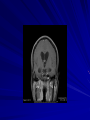
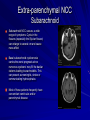
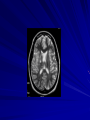
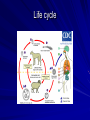
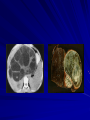
Trematodes (Flukes) and Cestodes Jose A. Serpa, M.D. Assistant Professor Baylor College of Medicine References The Travel and Tropical Medicine Manual by Elaine Jong and Christopher Sandford. Fourth Edition, 2008 Principles and Practices of Infectious Diseases. Mandel, Bennett and Dolin. Sixth Edition, 2005 DPDx. Laboratory Identification of Parasites of Public Health Concern (CDC). Second Edition, 2006. Special thanks to Dr. A.C. White Jr. for allowing me to use some of his slides Agenda 1. Trematodes (flukes): definition and classification 2. Schistosomes, liver flukes and lung flukes 3. Cestodes: Intestinal tapeworms 4. Cestodes: Tissue (cyst) stage (cysticercosis and ecchinococcosis) Cayetano Heredia National Hospital and Institute of Tropical Medicine Alexander von Humboldt, Lima , Peru 1. Trematodes Trematodes The flukes, or trematodes, are leaf-shaped parasites with a blind bifurcate intestinal tract Size varies from 1 mm to more than 10cm Most are hermaphroditic, except schistosomes Classification 1. Blood flukes (Schistosomes) 2. Hepatobiliary (Clonorchis sinensis, Opistorchis spp. and Fasciola hepatica) 3. Lung flukes (Paragonimus) 4. Intestinal flukes (Metagonymus, Heterophyes, Fasciolopsis) Eggs of trematodes From DPDx, website for laboratory diagnosis of parasitic diseases of the Division of Parasitic Diseases, National Centers for Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, [http://www.dpd.cdc.gov/DPDx/]. 2. A. Schistosomiasis Schistosomiasis Trematode infection caused by “blood flukes” which live in venules of the gastrointestinal and genitourinary tract There are approximately 200 million persons infected in the world (120 million are symptomatic and 100 000 die each year) It affects 74 countries (80% of cases occur in Sub-Saharan Africa) Human Schistosomes and their geographic distribution S. mansoni: Africa, Brazil, Middle East, Caribean S. haematobium: Africa, Middle East S. japonicum: China, Phillipines, SE Asia S. mekongi: Laos, Cambodia S. intercalatum: Central Africa (less common) Life cycle Cercariae penetrate human skin during contact or immersion in fresh water inhabited by infected snails Cercariae transform into schistosomulae , go into bloodstream and then migrate to liver and lung where they mature (4-6 weeks) Adult male and female pair and migrate to the Sup Mes vein (S. japonicum and S. mekongi), Inf Mesenteric vein (S. mansoni) or venous plexus sorrounding the bladder (S. hematobium) Eggs are excreted in feces or urine and deposited in fresh water. They hatch, miracidia emerge and infect snails of certain species. Cercariae emerge from infected snails Clinical syndromes Asymptomatic Schistosome (cercarial) dermatitis Acute Schistosomiasis Chronic Schistomiasis Schistosome dermatitis It is due to skin penetration of cercaria Pruritic maculopapular rash It can last from hours (first time exposed) to days (previously sensitized) Acute Schistosomiasis (Katayama fever) Occurs at the time of initial parasite egg-laying (hypersensitivity to egg- associated Ags) about 2-8 weeks after infection Consists of fever, rash/urticaria, cough, abdominal pain, diarrhea, hepatosplenomegaly and eosinophilia Usually in persons from non-endemic areas More common with S. mansoni and S. haematobium Chronic Schistosomiasis Intermittent dysentery and bowel obstruction Hepatosplenic disease Urinary tract disease Less common: Lung or CNS disease Hepatosplenic schistosomiasis Occurs in S. mansoni , S. japonicum and S. mekongi Pathogenesis: granulomas around embolized eggs that become trapped in small portal veins (liver) Initial stage: hepatomegaly Later stages: Periportal fibrosis blocks portal blood flow which leads to portal hypertension (splenic congestion, ascites and esophageal bleed) HBV or HCV coinfection worsens prognosis Urinary tract disease Occurs in S. haematobium infections Passage of eggs through the bladder wall cause hematuria and dysuria (inflammation and ulcerations) Granulomatous lesions with subsequent fibrosis of the bladder wall cause urinary reflux and obstruction (hydroureter, hydronephrosis, chronic bacteriuria, renal failure and squamous carcinoma of bladder) Lung and CNS schistosomiasis Eosinophilic pneumonitis results when eggs reaching the general circulation are trapped in the alveolar capillaries Aberrant migration of adult worms or embolization of eggs to the spinal cord or brain can cause transverse myelitis (S. mansoni or S. haematobium) or seizures (S. japonicum) respectively Keep in mind… Acute infection of humans with avian species of Schistosomes can result in an allergic skin reaction called swimmer’s itch (can not mature in the human host and die in the skin). NO need of antiparasitic therapy Pathogenic adult Schistosoma can persist in the human host for decades so infections can present in non endemic areas among immigrants from endemic regions An interesting association Prolonged and recurrent Salmonella bacteremia and/or bacteriuria has been reported in patients chronically infected with schistosomiasis Diagnosis Persons with a history of freshwater exposure in endemic areas (even if eosinophilia is absent) Identification of eggs in stool or urine samples Identification of eggs in tissues (e.g. rectal or bladder mucosa) Serologic tests (CDC): FAST-ELISA (99% sensitive) and immunoblot (species-specific) Imaging studies (US, CT, MRI) as clinically indicated Schistosoma eggs Schistosoma eggs in tissues Treatment Praziquantel is the drug of choice Dosing: 40mg/kg in 2 divided doses on a single day (S. mansoni and S. haematobium) or 60mg/kg in 3 divided doses 4-6h apart on a single day (S. Japonicum) Artemisinins (e.g. artemether) has antischistosomal as well as antimalarial activity Steroids can be used along with praziquantel for Katayama fever or CNS disease Prevention and control of Schistosomiasis Avoid contact with fresh water in endemic areas Annual mass drug administration of praziquantel Environmental measures to control the snails and promote sanitary disposal of human excrement (cost beyond the reach of poor countries) 2. B. Liver flukes Liver flukes They include: 1. Clonorchis sinensis 2. Opisthorchis sp. 3. Fasciola hepatica Clonorchis and Opisthorchis Largely found in the Far East, Southeast Asia and Russia The hermaphroditic adult worms measure 5 to 25mm x 2 to 5mm and inhabit the intrahepatic biliary ducts Humans become infected by eating raw or inadequately cooked fish Life cycle Clinical syndromes Asymptomatic eosinophilia RUQ abdominal pain with jaundice Acute biliary obstruction Acute pancreatitis Recurrent pyogenic cholangitis Cholangiocarcinoma Diagnosis and Treatment Based on identification of eggs in the stool or adult worms during surgery or ERCP Imaging studies (US, CT or MRI) can demonstrate biliary dilation Praziquantel is the drug of choice (75mg/kg in 3 divided doses 4-6h apart). Surgery or ERCP for acute biliary obstruction Fasciola hepatica Large liver fluke (adult worm measures 3.0 x 1.5 cm) that lives in the bile ducts of its mammalian hosts (humans, sheep and cattle) Reported in 61 countries from Europe, North Africa, Asia and South America (Bolivia and Peru) More than 3 million cases in the world Transmission Parasite eggs passed in stools hatch into miracidia which infects fresh water snails. Cercaria emerge from snails and attach to aquatic plants (encyst as metacercaria) Humans ingest contaminated aquatic plants (e.g. water cress) Metacercaria excyst, penetrate the intestinal wall, enter the peritoneum, and then pass through the liver capsule to the biliary tract Clinical syndromes 1. Acute phase Associated with parasite migration (duodenum – peritoneal cavity – liver) and burrowing through the liver Causes fever, abdominal pain, hepatomegaly and eosinophilia 2. Chronic phase Due to biliary involvement Intermittent “biliary colic”, cholangitis Diagnosis and management Serology for acute phase Demonstration of eggs in stools, bile or duodenal aspirates Imaging studies (US, CT) show linear hepatic tracts Treatment is with triclabendazole (10mg/kg once or twice). Also bithionol, nitazoxanide. 2. C. Lung flukes Lung flukes Most important species: Paragonimus westermani, P. heterotremus, P. skrjabini, P. miyazakii (Asia), P. africanus, P uterobilateralis (Africa), P. mexicana, P ecuadoriensis (South and Central America) Cases reported from Asia, Africa and Central and South America Life cycle Adult worms lay eggs that are coughed up in the sputum or swallow and passed in the stools Eggs hatch in freshwater and develop into miracidia that infects snails (cercaria) Cercaria infects freshwater crustaceans (metacercaria) Humans are infected when eating raw or inadequately cooked crabs or crayfish Metacercaria excyst in the duodenum peritoneum diaphragm pleura lung Clinical manifestations Pulmonary paragonimiasis Extrapulmonary paragonimiasis Pulmonary paragonimiasis Resembles pulmonary tuberculosis Initial stages: cough productive of brownish sputum with intermittent hemoptysis Chronic stages: profuse expectoration, dyspnea, hemoptysis, pleural effusions and pneumothorax CRX: infiltrates, cavities, cysts, nodules, pleural effusions Extrapulmonary paragonimiasis Ectopic migration of eggs from the bowel Most common: CNS, skin, liver, peritoneum CNS: brain lesions (seizures, headaches, visual disturbances), meningitis Skin: Migratory lesions (resemble cutaneous larva migrans) Diagnosis and treatment Identification of eggs in stools or sputum Gram or AFB stain in sputum may destroy eggs Serology: Immunoblot from CDC (Sen: 96%, Sp: 100%) Drug of choice is praziquantel (75mg/kg/day divided in 3 doses for 2 days) Adult flukes 3. A. Cestodes Intestinal tapeworms Tapeworms (adult stage) Tapeworms are segmented flat worms that have a head or scolex (which attaches to the intestine) and rhomboid-shaped segments termed proglottids The tapeworms elongates from each scolex by forming proglottids (which contain male and female reproductive organs). Adult tapeworms may reach lengths of 4.5 – 10m Clinically important tapeworms Tapeworm Common name Transmission Taenia solium Pork tapeworm Ingesting meat (pork) containing larvae Taenia saginata Beef tapeworm Ingesting meat (beef) containing larvae Dyphilobotrium sp. Fish tapeworm Ingesting fish containing larvae Hymenolepis sp. Dwarf tapeworm Accidental ingestion of fecal material (containing eggs) Dipyllidium caninum Dog tapeworm Accidental ingestion of dog fleas Life cycle: generalities The proglottids (containing eggs) shed periodically in the feces Eggs are ingested by an intermediate host - cow, pig, fish. Then the eggs hatch and larvae emerge, penetrate the gut and migrate to the tissues (especially the muscle) The lifecycle is completed when humans ingest encysted larvae- containing meat Epidemiology – T. solium is prevalent worldwide (60 million) but rare in US except in immigrants from endemic areas of Latin America and Asia – T. saginata is found worldwide (60 million), especially in Africa and Latin America. In the US, it is seen among people who eat raw or undercooked beef (e.g. steak tartare) – D. latum is prevalent worldwide (10 million) particularly among people who ingest raw, pickled, or marinated fish. D. latum in the US has been associated with sushi and sashimi made of raw pacific salmon and some Scandinavian pickled fish dishes (carp, herring, smelt) – H. nana and H. diminuta(dwarf, 2cm long) is prevalent worldwide (30 million) especially in children. Because of person to person spread, nurseries and day-care facilities are sites of transmission Life cycle of Dyphillobotrium Life cycle of Hymenolepis Life cycle of Dipylidium Clinical manifestations Most infections are asymptomatic or with minor abdominal symptoms. Patients may complain of passing worms D. latum can compete for absorption of vitamin B12 causing B12 deficiency. Diagnosis and treatment Eggs can be identified in the stools, but this may require repeated examinations The eggs of T. saginata and T. solium indistinguishable Gravid proglottids or the scolex can be examined to tell the species apart Treatment is praziquantel Tapeworm eggs Tapeworms 3. B. Cestodes Tissue cysts (larval stage) Cysticercosis It is a tissue infection (e.g. subcutaneous tissues, brain, eye, muscle, heart, etc) caused by the larval stage of Taenia solium. Neurocysticercosis = cysticercosis of the nervous system Cysticercosis is prevalent in Latin America, Sub-Saharan Africa, India, China and Southeast Asia – Serosurveys in endemic areas: up to 10% infection rates. Studies from India, Mexico, Peru, and Ecuador documented that up to half of patients with adult onset seizures had evidence of neurocysticercosis by imaging studies – In the US, NCC is primarily a disease of immigrants (Hispanics from Central America). Local transmission has been documented in the setting of household tapeworm carriers Life cycle of Taenia solium Pathophysiology There is typically an incubation period of several years before onset of clinical symptoms This quiescent phase results from the parasite’s ability to evade the host immune system. However as the cysticerci age, they lose this ability which leads to an intense inflammatory reaction (causing seizures) Over a few months, the inflamed lesion either resolves radiographically or is replaced by a small calcification Cysticerci in the cerebral ventricles often cause obstructive hydrocephalus as a result of mechanical obstruction Types of neurocysticercosis Neurocysticercosis can cause a wide spectrum of clinical forms depending on the number of parasites, their location, and the degree of host inflammation It can be broadly divided into: 1. Parenchymal: solitary lesion, multiple lesions 2. Extra-parenchymal disease: a. b. c. Intraventricular cysts Subarachnoid cysts Spinal cord cysts Parenchymal NCC A solitary degenerating parenchymal lesion is perhaps the most common presentation of neurocysticercosis worldwide Seizures are the main clinical manifestation of this type of NCC Other symptoms: headaches, neurologic focal deficits Extra-parenchymal NCC Intraventricular Intraventricular cysts typically present with increased intracranial pressure from obstructive hydrocephalus Headache, nausea, papilledema and altered mental status can also be present Extra-parenchymal NCC Subarachnoid Subarachnoid NCC causes a wide range of symptoms. Cysts in the fissures (especially the Sylvian fissure) can enlarge to several cm and cause mass effect Basal subarachnoid cysticercosis carries the worst prognosis since numerous cysticerci may fill the basilar cisterns leading to arachnoiditis. This can present as meningitis, stroke or communicating hydrocephalus Most of these patients frequently have concomitant ventricular and/or parenchymal disease Calcifications: inactive lesions? Diagnosis Imaging studies: MRI and CT Serology: Enzyme-linked immunoelectrodifusion transfer blot (EITB) More accurate in serum than in CSF. The assay has a nearly 100% specificity and 98% sensitivity for those with multiple cysticerci. (sensitivity is only 70% in patients with a single parenchymal cyst) Screening for taeniasis in patients or household contacts(?) Diagnostic criteria Absolute criteria: Histopathology, Parasites seen in eye, MRI with cyst and scolex Major criteria: CT/MRI consistent with NCC, +EITB, resolution (spontaneous or with ABZ/PZQ), single enhancing lesion <20 mm w/o Symptoms or signs of other disease Minor criteria: CT compatible, clinical manifestations c/w NCC, cysticercosis outside CNS (e.g. muscle calcifications) Epidemiologic criteria: Household contact of tapeworm carrier, residence or prolonged travel to endemic area Definitive diagnosis: one absolute criterion or two major plus one minor and one epidemiologic criterion 2. Probable diagnosis: one major plus two minor criteria, one major plus one minor plus one epidemiologic criteria, or three minor plus one epidemiologic criteria 1. Treatment Symptomatic therapy must be the initial goal of management in neurocysticercosis. (anti-epileptic medications, surgical CSF diversion procedures, and corticosteroids respectively) Antiparasitic medications also play an important although still controversial role in the treatment of NCC Surgery (VP shunts, open craniotomy and especially endoscopic removal of cysts) also play a role in selected cases Treatment consensus Viable or degenerating parenchymal cysts: AED, steroids, antiparasitics Intraventricular cysts: endoscopic removal of cysts or VPS followed by antiparasitics and steroids Subarachnoid: Steroids, antiparasitics (repeated courses?) +/- VPS Calcifications: AED (current rec’s against use of antiparasitics) Hydatid cyst It is an infection of liver and lung due to the encysted larva of the cestode Echinococcus spp. Two main species: 1. E. granulosus: Cystic hydatid disease. Endemic in sheep raising areas of the world (e.g South America, Middle East). There are small foci in the US (California, Utah and Alaska) 2. E. multilocularis: Alveolar hydatid disease. Endemic in Alaska, Central Europe and Northern Japan Dogs and wild canines are the definitive hosts (adult worms in intestines). Sheep and catle (E. granulosis) or rodents (E. multilocularis) are the intermediate hosts (hydatic disease) Life cycle Clinical manifestations and diagnosis Hydatid cyst disease (E. granulosus) presents with a slowly expanding mass lesion usually in the liver (60%) or lungs (25%). The most serious complications result from cyst rupture, which can cause anaphylaxis or numerous daughter lesions. Alveolar hydatid disease (E. multilocularis) presents with an expanding infiltrative process (90% in the liver). Diagnosis: Imaging studies (US, CT), serology (ELISA at CDC) is 83% sensitive Treatment In experienced centers, E. granulosus cysts are now mainly treated by PAIR procedure (percutaneous aspiration, injection of scolidical agents, and reaspirations). PAIR should be undertaken while on albendazole therapy (spilled cyst fluid can spread infection to other areas such as the peritoneal cavity) Chemotherapy alone may be attempted with albendazole for small or inoperable lesions Surgical removal is the treatment of choice for complicated cysts (e.g. communication with the biliary tract) and for E. multilocularis. Consider pre- and post-operative albendazole