* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download coord. chem2 – sb
Survey
Document related concepts
Transcript
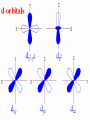
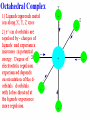
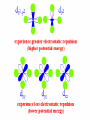
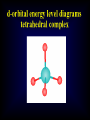
Coordination Chemistry Kvcrpf avadi Class xii SPECTROCHEMICAL SERIES List of ligands ranked in order of their ability to cause large orbital separations. A shortened list includes: I- < Br- < SCN- ~Cl- < F- < OH- ~ ONO- < C2O42- < H2O < NCS- < EDTA4- < NH3 ~ pyr ~ en < bipy < phen < CN- ~ CO Comparison of calculated spin-only magnetic moments with experimental data or some octahedral complexes Ion Config mso mobs Ti(III) d1 (t2g1) 3 = 1.73 B.M. 1.6-1.7 B.M. V(III) d2 (t2g2) Cr(III) d3 (t2g3) Cr(II) 2.7-2.9 d4 high spin (t2g3 eg1) 8 = 2.83 15 = 3.88 24 = 4.90 Cr(II) d4 low spin (t2g4) 8 = 2.83 3.2-3.3 Mn(II)/ Fe(III) d5 high spin (t2g3 eg2) 35 = 5.92 5.6-6.1 Mn(II)/ Fe(III) d5 low spin (t2g5) 3 = 1.73 1.8-2.1 Fe(II) d6 high spin (t2g4 eg2) 24 = 4.90 5.1-5.7 Co(II) d7 high spin (t2g5 eg2) 15 = 3.88 4.3-5.2 Co(II) d7 low spin (t2g6 eg1) 3 = 1.73 1.8 Ni(II) d8 (t2g6 eg2) 8 = 2.83 2.9-3.3 Cu(II) d9 (t2g6 eg3) 3 = 1.73 1.7-2.2 3.7-3.9 4.7-4.9 Comparison of calculated spin-only magnetic moments with experimental data for some tetahedral complexes Ion Config mso mobs Cr(V) d1 (e1) 3 = 1.73 B.M. 1.7-1.8 B.M. Cr(IV) / Mn(II) d2 (e2) 8 = 2.83 2.6 - 2.8 Fe(V) d3 (e2 t21) 15 = 3.88 3.6-3.7 - d4 (e2 t22) 24 = 4.90 - Mn(II) d5 (e2 t23) 35 = 5.92 5.9-6.2 Fe(II) d6 (e3 t23) 24 = 4.90 5.3-5.5 Co(II) d7 (e4 t23) 15 = 3.88 4.2-4.8 Ni(II) d8 (e4 t24) 8 = 2.83 3.7-4.0 Cu(II) d9 (e4 t25) 3 = 1.73 - Thermodynamic Stability In the laboratory course, it will have been pointed out that the "stability of a complex in solution" refers to the degree of association between the two species involved in the state of equilibrium. Qualitatively, the greater the association, the greater the stability of the compound. The magnitude of the (stability or formation) equilibrium constant for the association, quantitatively expresses the stability. Thus, if we have a reaction of the type: M + 4L -> ML4then the larger the stability constant, the higher the proportion of ML4 that exists in the solution. Free metal ions rarely exist in solution so that M, will usually be surrounded by solvent molecules which will compete with the ligand molecules, L, and be successively replaced by them. For simplicity, we generally ignore these solvent molecules and write four stability constants as follows: l. M + L -> ML K1 = [ML] / [M] [L] 2. ML + L -> ML2 K2 = [ML2] / [ML] [L] 3. ML2 + L -> ML3 K3 = [ML3] / [ML2] [L] 4. ML3 + L -> ML4 K4 = [ML4] / [ML3] [L] where K1, K2 etc. are referred to as "stepwise stability constants". Alternatively, we can write the "Overall Stability Constant" thus: M + 4L -> ML4 b4 = [ML4]/ [M] [L]4The stepwise and overall stability constants are therefore related as follows: b4 =K1.K2.K3.K4 or more generally, bn =K1.K2.K3.K4--------------K nIf we take as an example, the steps involved in the formation of the cuprammonium ion, we have the following: Cu2+ + NH3 <-> Cu(NH3)2+ K1 = [Cu(NH3)2+]/[Cu2+] [NH3] CuNH32+ + NH3 <-> Cu(NH3)22+ K2 = [Cu(NH3)22+]/[Cu(NH3)2+] [NH3] etc. where K1, K2 are the stepwise stability constants. Also: b4 = [Cu(NH3)42+]/[Cu2+] [NH3]4 The addition of the four ammine groups to copper shows a pattern found for most formation constants, in that the successive stability constants decrease. In this case, the four constants are: logK1 =4.0, logK2 =3.2, logK3 =2.7, logK4 =2.0 or logb4 =11.9 A number of texts refer to the instability constant or the dissociation constant of coordination complexes. This value corresponds to the reciprocal of the formation constant, since the reactions referred to are those where fully formed complexes break down to the aqua ion and free ligands. This should be compared with the equation for the formation constant given earlier. So, it is usual to represent the metal-binding process by a series of stepwise equilibria which lead to stability constants that may vary numerically from hundreds to enormous values such as 1035 and more. For this reason, they are commonly reported as logarithms. It is additionally useful to use logarithms, since log(K) is directly proportional to the free energy of the reaction. DG° = -RTLnb DG° = -2.303 RTLog10b DG° = DH° - TDS° For a problem relating to metal complex formation and calculations of free metal ions concentrations, try your hand at CALCULATION # ONE. Other problems can be found in the Tutorial paper for this course. The Chelate Effect The chelate effect can be seen by comparing the reaction of a chelating ligand and a metal ion with the corresponding reaction involving comparable monodentate ligands. For example, comparison of the binding of 2,2'-bipyridine with pyridine or 1,2-diaminoethane (ethylenediamine=en) with ammonia. It has been known for many years that a comparison of this type always shows that the complex resulting from coordination with the chelating ligand is much more thermodynamically stable. This can be seen by looking at the values for adding two monodentates compared with adding one bidentate, or adding four monodentates compared to two bidentates, or adding six monodentates compared to three bidentates. Uses of Coordination Compounds A brief survey of some of the uses of coordination compounds includes: l. Dyes and Pigments: Coordination compounds have been used from the earliest times as dyes and pigments, for example madder dye which is red, was used by the ancient Greeks and others. It is a complex of Hydroxyanthraquinone. A more modern example is the pigment copper phthalocyanine, which is blue. Prussian Blue, Khaki Phalocyanin complexes of several metal ions are used as LASER dyes. 2. Analytical Chemistry: You have already encountered many such uses during the laboratory course. (a) Colour Tests: Since many complexes are highly coloured they can be used as colourimetric reagents e.g. formation of red 2,2'-bipyridyl and l,l0-phenanthroline complexes as a test for Fe2+ (b) Gravimetric Analysis: Here chelating ligands are often used to form insoluble complexes e.g. Ni(DMG)2 and Al(oxine)3 (see laboratory manual). (c) Complexometric Titrations and Masking Agents: An example of this is the use of EDTA in the volumetric determination of a wide variety of metal ions in solution, e.g. Zn2+, Pb2+, Ca2+,Co2+, Ni2+, Cu2+, etc. By careful adjustment of the pH and using suitable indicators, mixtures of metals can be analysed, e.g. Bi3+ in the presence of Pb2+ (see laboratory manual). Alternatively, EDTA may be used as a masking agent to remove a metal ion which would interfere with the analysis of a second metal ion present. 3. Sequestering Agents: Related to their use as masking agents is the use of ligands for "sequestering" i.e. for the effective removal of objectionable ions from solution in industrial processing, e.g. EDTA is used to "soften" water. The addition of EDTA to water is used in boilers etc., to prevent "scaling" or build up of insoluble calcium salts. 4. Extraction of Metals: certain metals can be leached from their ores by formation of stable complexes e.g. Ag and Au as complexes of cyanide ion. 5. Bio-Inorganic Chemistry: Naturally occurring complexes include haemoglobin, chlorophyll, vitamin B12 etc. EDTA and other complexing agents have been used to speed the elimination of harmful radioactive and other toxic elements from the body. (e.g. Pb2+). In these cases a soluble metal chelate is formed. 6. Medicine: Radiodiagnostics: Complexes of radioactive Co, Ge, Tc, Tl,Hg, Yb, In, I MRI: Complexes of Gd(III), Fe(III), Mn(II) Chemo-therapy/Anti-tumor: cis-Platin, carboplatin, spirogermanium Rhumatoid Arthitis: Auranofin, Sanocrysin, Myocrysin, Solganol