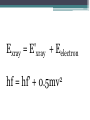* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 1 – Foundations of Quantum Theory
Casimir effect wikipedia , lookup
Hydrogen atom wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Double-slit experiment wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Delayed choice quantum eraser wikipedia , lookup
Planck's law wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Particle in a box wikipedia , lookup
Renormalization wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Atomic theory wikipedia , lookup
Matter wave wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
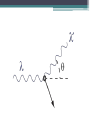
Foundations of Quantum Theory Maxwell Planck • 1894 – Turned his attention to Black Body Radiation The Problem According to Kirchoff • “how does the intensity (Energy/(second x unit area)) of the electromagnetic radiation emitted by a black body (a perfect absorber) depend on the frequency of the radiation (i.e., the color of the light) and the temperature of the body?” • Planck used a mathematical interpretation of the problem to predict the physical application • His math matched the experimental results perfectly (Now that’s good Physics!!!) • The conclusion of the research (not his conclusion) was interpreted to be that the energy could only be released in quantised (packet) form now referred to as a photon Planck's Quantum idea: challenged the classical wave theory of light by proposing that electromagnetic waves do not transmit energy in a continuous manner, but instead, energy is transmitted in small packages or bundles. Planck's Quantum idea: challenged the classical physics of Newton, since it proposed that an object is not free to vibrate with any random energy; the energy is restricted to certain discrete values. • "My unavailing attempts to somehow reintegrate the action quantum into classical theory extended over several years and caused me much trouble." - Max Planck • Classical theory views light as a wave • Some argue that the father of Quantum theory was Einstein because Planck didn’t really realize what the math said (he kept trying to make light a wave when his calculations seem to refer to a packet of energy whose size varies with frequency) E = hf • h – Planck’s Constant h= 6.626 x 10-34 m2kg/s • f – frequency of the photon (Hz) Reminder: f=c/ • This formula states that the energy released by an object at a certain frequency is a multiple of the energy of each packet of light (the frequency by Planck’s constant) Blackbody Radiation 1. At a given temperature, a spectrum of different wavelengths is emitted, of varying intensity, but there is a definite intensity maximum at one particular wavelength. 2. As the temperature increases the intensity maximum shifts to a shorter wavelength (higher frequency) Photoelectric Effect What is it? • effect which involves electrons being separated from a substance as the substance is exposed to electromagnetic radiation. Condition for the Photoelectric Effect • When a photon hits a material and that energy is absorbed by an electron: ▫ If the energy absorbed is greater than the electron binding energy (energy holding it there) then the electron takes off ▫ We sometimes call these photoelectrons Note: • Some substances just reemit the radiation while some will dislodge electrons • If you increase the intensity of the light (more photons), the number of electrons ejected will increase but not the energy given each electron • Any extra energy is converted to the kinetic energy of the particle •Ephoton = W + Ek Compton Effect • The Compton Effect: The scattering of low energy photons after colliding electrons with high-energy photons • The scattering photons have lower energy and lower frequency • High energy photons are usually Xrays • Compton attributed particle like momentum to high energy photons Reinforces particle nature of photons • Attributes a momentum like property to photons • Since momentum is generally reserved for particles, photons act like particles ▫ Therefore, we can apply conservation of momentum and specifically cons of momentum for an elastic collision • Compton’s genius was to give light momentum by using mass-energy equivalence 𝑝𝑝ℎ𝑜𝑡𝑜𝑛 ℎ = 𝜆 Exray = E'xray + Eelectron hf = hf' + 2 0.5mv Pair Production Using Mass Energy Equivalence • When a photon collides with a heavy nucleus, the photon disappears • Since energy is equivalent to mass, two particles are produced with equal mass but opposite charge ▫ An electron and a positron