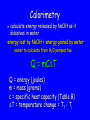* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Energy - Chemistry
Open energy system models wikipedia , lookup
Kinetic energy wikipedia , lookup
100% renewable energy wikipedia , lookup
Compressed air energy storage wikipedia , lookup
Public schemes for energy efficient refurbishment wikipedia , lookup
Energy storage wikipedia , lookup
World energy consumption wikipedia , lookup
Regenerative brake wikipedia , lookup
Low-Income Home Energy Assistance Program wikipedia , lookup
Energy Charter Treaty wikipedia , lookup
Zero-energy building wikipedia , lookup
International Energy Agency wikipedia , lookup
Low-carbon economy wikipedia , lookup
Energy policy of the United Kingdom wikipedia , lookup
Energy policy of Finland wikipedia , lookup
Alternative energy wikipedia , lookup
Energy efficiency in transport wikipedia , lookup
Distributed generation wikipedia , lookup
Energy returned on energy invested wikipedia , lookup
Micro combined heat and power wikipedia , lookup
Energy harvesting wikipedia , lookup
Gibbs free energy wikipedia , lookup
Environmental impact of electricity generation wikipedia , lookup
Negawatt power wikipedia , lookup
Internal energy wikipedia , lookup
Energy in the United Kingdom wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
Energy efficiency in British housing wikipedia , lookup
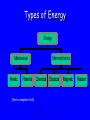
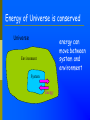
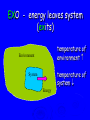
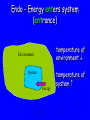
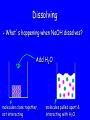
Energy Chapter 16 Energy: Ability to do Work Potential Energy (PE) = Energy of position aka STORED energy Kinetic Energy (KE) = Energy of motion Radiant Energy = Electromagnetic radiation Ex: Sunlight Types of Energy Energy Mechanical Kinetic Potential (Not a complete list!) Non-mechanical Chemical Electrical Magnetic Radiant Units of Energy SI 1 system - unit of energy is JOULE (J) Joule ≅ amount of energy required to lift 1 golf ball about 1 meter other energy units: calorie, Calorie, BTU 1 calorie = 4.18 Joules 1 Calorie = 1000 calories = 1 kilocalorie Kinetic Energy KE So = ½ x mass x velocity2 = ½ mv2 KE of matter depends on: how heavy and how fast Potential Energy Stapler Rubberband popper anything can have PE = energy of position = stored energy PE can be converted to KE Magnets PE in the system of 2 magnets depends on their relative position when magnets close together they will pull together due to attraction when magnets far apart they can’t attract each other Electromagnetic Radiation Sunlight – Visible radiation Ultraviolet radiation Infrared radiation Gamma rays X-rays Microwaves Radiowaves Applet spectrum Energy in Chemistry chemical energy is energy stored within chemical bonds heat is form of energy that flows from warmer object to cooler object (Macroscopic) Heat Energy heat: energy associated with motion of atoms/molecules in matter (Microscopic) symbol for heat energy = Q or q Heat Energy heat depends on amount of substance present we can only measure heat changes Temperature = measure of average KE of particles in substance Swimming pool of water vs. mug of water temperature is NOT energy temperature does NOT depend on amount of substance; energy does Law of Conservation of Energy energy is neither created nor destroyed in ordinary chemical or physical change energy before = energy after Energy can be converted from one form to another - potential to kinetic - radiant to electric - electric to heat - chemical to kinetic - chemical to electrical All physical & chemical changes are accompanied by change in energy The chemistry of energy changes is known as Thermochemistry! Energy Transfer measure changes in heat amount energy transferred from one substance to another can measure energy lost somewhere or energy gained somewhere else cannot measure absolute heat content of system Energy of Universe is conserved Universe EnvironmentEnvironment System Energy energy can move between system and environment Exothermic Change system releases heat to environment what happens to temperature of environment? EXO - energy leaves system (exits) what happens to energy level of system? what happens to temperature of system? EXO - energy leaves system (exits) temperature of environment Environment temperature of system System Energy Exothermic Change system: net energy loss! environment: net energy gain! energy lost = energy gained Endothermic Change system absorbs heat from environment what happens to temperature of environment? Endo what - Energy enters system happens to energy level of system what happens to temperature of system? Endo - Energy enters system (entrance) temperature of environment Environment System Energy temperature of system Endothermic Change system - net energy gain! environment - net energy loss! energy lost = energy gained Heat Flow heat flows from hotter object to cooler object cold pack on leg: heat flows from leg to cold pack! leg cools down; cold pack warms up Quantity of heat transferred quantity (amount) of heat transferred depends on temperature change mass of substance specific Heat of substance Calculating Heat Transferred simple system: •pure substance in single phase •calculate heat gained or lost using: Q = mCT Q = amount of heat transferred m = mass of substance C = specific heat capacity of the substance. T = temperature change = Tfinal – Tinitial Specific Heat amount heat energy required to raise temp of 1 gram of substance by 1oC symbol =c specific heat = a physical constant unique for each pure substance Calorimeter: used to measure heat changes other examples source Calorimetry changes in heat energy are measured by calorimetry “universe” contained in styrofoam cup “enviroment” “system” is water**** is whatever put in water Calorimetry energy lost = energy gained difficult to monitor “system” easy to monitor “environment” (water) energy lost/gained by environment = energy gained/lost by system Calorimetry 10 grams of NaOH is dissolved in 100 g of water & the temperature of the water increases from 22C to 30C. was dissolving process endothermic or exothermic how do you know? Exothermic – temperature of environment ↑ Dissolving What’s happening when NaOH dissolves? Add H2O molecules close together, not interacting molecules pulled apart & interacting with H2O Calorimetry calculate energy released by NaOH as it dissolves in water energy lost by NaOH = energy gained by water • easier to calculate from H2O perspective Q = mCT Q = energy (joules) m = mass (grams) c = specific heat capacity (Table B) T = temperature change = Tf - Ti Calorimetry & Q = mCT temperature 22C to 30C 30C what of water increased from -22C = 8C = T mass to use? temp change was for water, so want mass of water m = 100 g same goes for specific heat capacity; calculate heat absorbed by water cH 0 = 4.18J/g 2 Q = mCT Q = (100 g)(4.18 J/g)(8C) Q = 3344 Joules Stability and Energy if energy is high, stability is low if energy is low, stability is high