* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Drawing Organic Structures Functional Groups Constitutional Isomers
Persistent carbene wikipedia , lookup
2-Norbornyl cation wikipedia , lookup
Asymmetric induction wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Homoaromaticity wikipedia , lookup
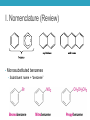
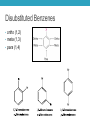
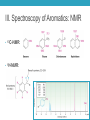
AROMATIC COMPOUNDS Dr. Sheppard CHEM 2412 Summer 2015 Klein (2nd ed.) sections: 18.1, 18.2, 18.8, 18.3, 18.4, 18.5 Aromatic Compounds • Originally distinguished because of smell • Then noticed trends in reactivity • Now, highly unsaturated, stable compounds • Unreactive to many reagents that react with alkenes • Aromatic hydrocarbons = arenes (Ar-) • Most famous is benzene Aromatic Compounds I. II. III. IV. V. Nomenclature Review Physical Properties Spectroscopy Benzene Structure Aromaticity I. Nomenclature (Review) • Monosubstituted benzenes • Substituent name + “benzene” Common Benzene Compounds Benzene Nomenclature • If substituent has greater than 6 carbons, it becomes the parent, and benzene is called a phenyl group • Benzene substituents: (Ph‒ or F‒) Disubstituted Benzenes • ortho (1,2) • meta (1,3) • para (1,4) Naming Disubstituted Benzenes • If one substituent is part of a common name, that name is the parent and that substituent is at carbon 1 • If neither substituent is part of a common name, list the substituents in alphabetical order (first alphabetically is at carbon 1) • If both substituents are part of common name, use this order of priority to determine the parent name: -CO2H > -CHO > -OH > -NH2 > -CH3 Naming Polysubstituted Benzenes • With 3 or more substituents do not use ortho, meta, para • Number ring to give smallest set of numbers • If a common name, use as parent (substituent at carbon 1) • List substituents in alphabetical order II. Physical Properties • Melting point • Based on “packing” • Benzene packs easily, so has a higher mp than other hydrocarbons • Substituted benzenes: para > ortho and meta due to packing • Boiling point • Polarity depends on substituents • Higher polarity = higher boiling point mp (°C) -17 -25 54 bp (°C) 181 173 170 III. Spectroscopy of Aromatics: IR • sp2 C-H absorption at 3030 cm-1 • Ring absorptions at 1450-1600 and 1660-2000 cm-1 • Also peaks in fingerprint region can differentiate substitution pattern III. Spectroscopy of Aromatics: NMR • 13C-NMR: • 1H-NMR: IV. Benzene Structure and Stability • Cyclic, planar, hexagonal shape • Conjugated • Hybridization of carbons? • Bond angles? • All H’s are identical • All C-C bonds are equivalent • Bond order = 1.5 Benzene Reactivity • Unsaturated, but doesn’t behave like alkene • Alkenes: • Benzene: • Benzene will reduce at high pressure and temperature or with special catalyst: Explanation for Benzene Stability 1. Molecular orbital model • Bonding and antibonding molecular orbitals • Skip this 2. Resonance model • Lots of orbital overlap and conjugation = very stable Kekulé structures Hybrid structure V. Aromaticity • All aromatic structures are similar in stability and reactivity • All have structural similarities • Example: Benzene is aromatic • But, 1,3-cyclobutadiene is not aromatic • Even though it resembles benzene and is resonance-stabilized • Reacts like an alkene (addition) not benzene (substitution) Criteria for Aromaticity • Hückel • Based on molecular orbital calculations 1. Cyclic 2. Planar 3. Unhybridized p orbital on each atom of the ring 4. (4n + 2) electrons in the p orbitals n 4n + 2 0 2 1 6 2 10 3 14 4 18 Examples • Benzene 1. Is it cyclic? 2. Is it planar? 3. Does it have an unhybridized p orbital on each atom of the ring? 4. How many electrons does it have in the p orbitals? Is that equal to (4n + 2)? Examples • [14] annulene • [#] is number of atoms in ring • Annulene = cyclic, conjugated hydrocarbon 1. Is it cyclic? 2. Is it planar? 3. Does it have an unhybridized p orbital on each atom of the ring? 4. How many electrons does it have in the p orbitals? Is that equal to (4n + 2)? Aromaticity • Molecules that are not aromatic (do not satisfy the 4 criteria listed above) are either: • Antiaromatic • Nonaromatic • The reactivity of both antiaromatic and nonaromatic molecules will be like alkenes (addition reactions) Antiaromatic 1. Cyclic 2. Planar 3. Unhybridized p orbital on each atom of the ring 4. (4n) electrons in the p orbitals • Examples: Nonaromatic • Either not cyclic, or not conjugated, or not planar • Examples: • Not cyclic: • Not conjugated: • Not planar: Aromaticity of Ions • Cyclopentadienyl cation • Cyclic • Planar • Unhybridized p orbitals • 4 electrons = 4n • Antiaromatic • Cyclopentadienyl anion • Cyclic • Planar • Atom with : can become sp2 hybridized • Lone pair in p orbital • Unhybridized p orbitals • 6 electrons = 4n+2 • Aromatic • Very stable, likely to form (H lost is more acidic than other hydrocarbon H’s) • Cyclopentadiene pKa = 16; cyclopentane pKa > 50 Aromaticity of Heterocycles • Heteroatoms with lone pairs • Determine which electrons are part of aromatic system • Examples: • Pyridine • Furan • Remember aromatic amines are weaker bases than aliphatic amines • Partially due to resonance delocalization • Also due to lone pair electrons counting toward aromaticity • Pyrrole loses aromaticity when protonated • What about pyridine? Polycyclic aromatic compounds • Fused ring systems • Naphthalene • Indole • Fused benzene rings Aromatic, antiaromatic, or nonaromatic?