* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Origin of the Earth`s Atmosphere - The Building Blocks For Learning
Survey
Document related concepts
Transcript
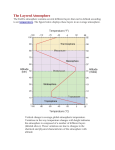
Origin of the Earth's Atmosphere Introduction Early Earth would have been very different and inhospitable compared to the Earth today. Hot Why? - Primordial heat, collisions and compression during accretion, decay of short-lived radioactive elements o Consequences - Constant volcanism, surface temperature too high for liquid water or life as we know it, molten surface or thin, unstable basaltic crust. Atmosphere - early atmosphere probably completely different in composition (H 2, He) Cooling o o o o o Primordial heat dissipated to space Condensation of water (rain), accumulation of surface water. Accumulation of new atmosphere due to volcanic out gassing Conditions appropriate for evolution of life Evolution of the Atmosphere Atmosphere - Envelope of gases that surrounds the Earth. Used by life as a reservoir of chemical compounds used in living systems. Atmosphere has no outer boundary, just fades into space. Dense part of atmosphere (97% of mass) lies within 30 km of the Earth (so about same thickness as continental crust). Chemical Composition Today - Nitrogen (N2)- 78%, Oxygen (O2)- 21%, Carbon Dioxide (CO2) 0.03 %, plus other miscellaneous gases (H2O for one). (figure and table from Lutgens and Tarbuck, The Atmosphere, 8th edition) First Atmosphere Composition - Probably H2, He These gases are relatively rare on Earth compared to other places in the universe and were probably lost to space early in Earth's history because o Earth's gravity is not strong enough to hold lighter gases o Earth still did not have a differentiated core (solid inner/liquid outer core) which creates Earth's magnetic field (magnetosphere = Van Allen Belt) which deflects solar winds. Once the core differentiated the heavier gases could be retained Composition of Gases of Second Atmosphere Second Atmosphere Produced by volcanic out gassing. Gases produced were probably similar to those created by modern volcanoes (H2O, CO2, SO2, CO, S2, Cl2, N2, H2) and NH3 (ammonia) and CH4 (methane) No free O2 at this time (not found in volcanic gases). Gases trapped in the heat from the sun and the crust which caused the atmosphere to warm up. Ocean Formation - As the Earth cooled, H2O produced by out gassing could exist as liquid in the Early Archean, allowing oceans to form. o Evidence - pillow basalts, deep marine beds in greenstone belts. Addition of O2 to the Atmosphere Today, the atmosphere is ~21% free oxygen. How did oxygen reach these levels in the atmosphere? Revisit the oxygen cycle: Oxygen Production o o o o Photochemical dissociation - breakup of water molecules by ultraviolet Produced O2 levels approx. 1-2% current levels Over millions of years, oxygen concentrations increased and moved to the upper atmosphere. UV light from the sun transformed O2 into O3 (Ozone). At these levels O3 (Ozone) can form to shield Earth surface from UV Photosynthesis - CO2 + H2O + sunlight = organic compounds + O2 - produced by cyanobacteria/stromatolites, and eventually higher plants - supplied the rest of O2 to atmosphere. Thus plant populations increased. When the CO2 levels decreased, the temperature began to lower. Temperature would decrease so much that it created an ice age. Oxygen Consumers o o o Chemical Weathering - through oxidation of surface materials (early consumer) Animal Respiration (much later) Burning of Fossil Fuels (much, much later) Throughout the Archean there was little to no free oxygen in the atmosphere (<1% of presence levels). What little was produced by cyanobacteria/stromatolites, was probably consumed by the weathering process. Once rocks at the surface were sufficiently oxidized, more oxygen could remain free in the atmosphere. During the Proterozoic the amount of free O2 in the atmosphere rose from 1 - 10 %. Most of this was released by cyanobacteria, which increase in abundance in the fossil record 2.3 Ga. Present levels of O2were probably not achieved until ~400 Ma. Evidence from the Rock Record Iron (Fe) i s extremely reactive with oxygen. If we look at the oxidation state of Fe in the rock record, we can infer a great deal about atmospheric evolution. Archean - Find occurrence of minerals that only form in non-oxidizing environments in Archean sediments: Pyrite (Fools gold; FeS2), Uraninite (UO2). These minerals are easily dissolved out of rocks under present atmospheric conditions. Banded Iron Formation (BIF) - Deep water deposits in which layers of iron-rich minerals alternate with iron-poor layers, primarily chert. Iron minerals include iron oxide, iron carbonate, iron silicate, iron sulfide. BIF's are a major source of iron ore, b/c they contain magnetite (Fe 3O4) which has a higher iron-to-oxygen ratio than hematite. These are common in rocks 2.0 - 2.8 B.y. old, but do not form today. Red beds (continental siliciclastic deposits) are never found in rocks older than 2.3 B. y., but are common during Phanerozoic time. Red beds are red because of the highly oxidized mineral hematite (Fe2O3), that probably forms secondarily by oxidation of other Fe minerals that have accumulated in the sediment. Conclusion - amount of O2 in the atmosphere has increased with time. Biological Evidence Chemical building blocks of life could not have formed in the presence of atmospheric oxygen. Chemical reactions that yield amino acids are inhibited by presence of very small amounts of oxygen. Oxygen prevents growth of the most primitive living bacteria such as photosynthetic bacteria/stromatolites, methane-producing bacteria and bacteria that derive energy from fermentation. Conclustion - Since today's most primitive life forms are anaerobic, the first forms of cellular life probably had similar metabolisms. Today these anaerobic life forms are restricted to anoxic (low oxygen) habitats such as swamps, ponds, and lagoons. Atmospheric oxygen built up in the early history of the Earth as the waste product of photosynthetic organisms and by burial of organic matter away from surficial decay. This history is documented by the geologic preservation of oxygen-sensitive minerals, deposition banded iron formations, and development of continental "red beds" or BIFs. Figure from the University of Michigan's Introduction to Global Change web site. Atmospheric Structure Not only does the atmosphere have a relatively stable composition, but it also has a structure to it. Gravity pushes the layers of air down on the earth's surface. The heavier gases move toward the Earth's surface and the lighter gases are in the higher altitudes. Troposphere The troposphere is the atmospheric layer closest to the planet and contains the largest percentage (around 80%) of the mass of the total atmosphere. Temperature and water vapor content in the troposphere decrease rapidly with altitude. Water vapor plays a major role in regulating air temperature because it absorbs solar energy and thermal radiation from the planet's surface. The troposphere contains 99 % of the water vapor in the atmosphere. Water vapor concentrations vary with latitude. All weather phenomena occur within the troposphere, although turbulence may extend into the lower portion of the stratosphere. Stratosphere The stratosphere is the second major strata of air in the atmosphere. It extends above the tropopause to an altitude of about 30 miles (50 km) above the planet's surface. The air temperature in the stratosphere remains relatively constant up to an altitude of 15 miles (25 km). Then it increases gradually to up to the stratopause. Because the air temperature in the stratosphere increases with altitude, it does not cause convection and has a stabilizing effect on atmospheric conditions in the region. Ozone plays the major role in regulating the thermal regime of the stratosphere, as water vapor content within the layer is very low. Temperature increases with ozone concentration. Solar energy is converted to kinetic energy when ozone molecules absorb ultraviolet radiation, resulting in heating of the stratosphere. The ozone layer is centered at an altitude between 10-15 miles (15-25 km). Approximately 90 % of the ozone in the atmosphere resides in the stratosphere. Ozone concentration in the this region is about 10 parts per million by volume (ppmv) as compared to approximately 0.04 ppmv in the troposphere. Ozone absorbs the bulk of solar ultraviolet radiation in wavelengths from 290 nm - 320 nm (UV-B radiation). These wavelengths are harmful to life because they can be absorbed by the nucleic acid in cells. Increased penetration of ultraviolet radiation to the planet's surface would damage plant life and have harmful environmental consequences. Appreciably large amounts of solar ultraviolet radiation would result in a host of biological effects, such as a dramatic increase in cancers. Other Graphs