* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The complexities of Varicella Zoster Virus infection: problems for
Vectors in gene therapy wikipedia , lookup
2015–16 Zika virus epidemic wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Transmission and infection of H5N1 wikipedia , lookup
Marburg virus disease wikipedia , lookup
Canine distemper wikipedia , lookup
Canine parvovirus wikipedia , lookup
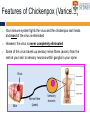
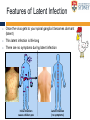
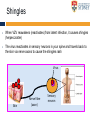
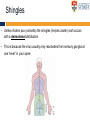
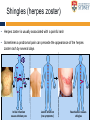
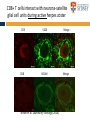
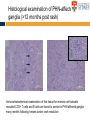
The complexities of Varicella Zoster Virus infection: problems for vaccination and therapy Infectious Diseases & Immunology Varicella Zoster Lab University of Sydney Associate Professor Allison Abendroth [email protected] Human Herpesviruses Alphaherpesviruses • Varicella Zoster Virus • Herpes Simplex Virus 1 • Herpes Simplex Virus 2 Betaherpesviruses • Cytomegalovirus • Human Herpesvirus 6 • Human Herpesvirus 7 Gammaherpesviruses • Epstein-Barr Virus • Kaposi’s Sarcoma-associated Herpesvirus Shared characteristics Large dsDNA genomes Virion structure Lytic and latent life cycles Extensive immune evasion strategies Varicella Zoster Virus (VZV) Varicella zoster virus (VZV) causes two different diseases: (1) chickenpox (varicella) (2) shingles (herpes zoster) The same virus causes both diseases Features of Chickenpox (Varicella) • When you get infected with VZV for the first time, the virus causes a widespread blistering rash called chickenpox (varicella) • It is likely that dendritic cells in the respiratory mucosa become infected with VZV and transfer to T cell in the draining lymph node • T cells then disseminate VZV to distant cutaneous sites, causing the widespread rash Initial infection causes chicken pox Features of Chickenpox (Varicella) • >90% of chickenpox occurs in children 1-14 yrs of age • Chickenpox is a relatively mild disease in healthy children • Complications and significant disease can occur in older age groups, the fetus, new born infants and is life-threatening in immunocompromised people Chicken AND Egg Pox How does VZV spread to others during chickenpox? • Chickenpox is a very contagious disease • The virus spreads very easily from people with chickenpox to others who have never had the disease • The virus spreads in the air when an infected person coughs or sneezes Features of Chickenpox (Varicella) Your immune system fights the virus and the chickenpox rash heals and most of the virus is eliminated However, the virus is never completely eliminated Some of this virus travels up sensory nerve fibres (axons) from the rash at your skin to sensory neurons within ganglia in your spine Virus Skin Nerve fibre (axon) Sensory neuron Features of Latent Infection Once the virus gets to your spinal ganglia it becomes dormant (latent) This latent infection is life-long There are no symptoms during latent infection Initial infection causes chicken pox Latent infection (no symptoms) Shingles When VZV reawakens (reactivates) from latent infection, it causes shingles (herpes zoster) The virus reactivates in sensory neurons in your spine and travels back to the skin via nerve axons to cause the shingles rash Virus Skin Nerve fibre (axon) Sensory neuron Shingles • Unlike chicken pox (varicella) the shingles (herpes zoster) rash occurs with a dermatomal distribution • This is because the virus usually only reactivates from sensory ganglia at one “level” in your spine Shingles (herpes zoster) • Herpes zoster is usually associated with a painful rash • Sometimes a prodromal pain can precede the appearance of the herpes zoster rash by several days Initial infection causes chicken pox Latent infection (no symptoms) Reactivation causes shingles Shingles • Overall ~20% of people who had chickenpox get shingles • Chances of shingles increases dramatically with age • Immunocompromised people are at higher risk for shingles • The blisters that form typically scab over in 7-10 days and heal within 2-4 weeks Day 1 Day 2 Day 5 Day 6 Can VZV be spread from someone with shingles to someone else? • The virus is spread through direct contact with fluid from the rash blisters, not through sneezing or coughing • Shingles is less contagious than chickenpox • A person with shingles can spread the virus when the rash is in the blister-phase VZV and Neuropathic Pain • Acute pain can last throughout herpes zoster • Burning pain, itching, oversensitivity, "pins and needles”, tingling, pricking sensation • Pain may be mild to extreme • In addition to pain during herpes zoster, for some people the problem persists well beyond rash resolution… Most common complication of herpes zoster is post-herpetic neuralgia (PH • Herpes zoster is sometimes followed by a severe pain syndrome called post herpetic neuralgia (PHN) • 20-60% of people with herpes zoster will experience PHN after the rash resolves • PHN also increases with age • PHN can last for months or even years • Often severe, debilitating nerve pain which has a major negative impact on quality of life Treatment strategies for post-herpetic neuralgia • Currently there is no cure for PHN • Current treatment options to ease PHN pain include: • Gabapentin, lidocaine patch, opioid analgesics, pregabalin and tricyclic antidepressants, corticosteriods (anti-inflammatories) • Anti-viral approaches (eg. Acyclovir, famciclovir, valacyclovir) are designed to inhibit viral replication and hasten arrest of viral shedding and increase rash healing and reduce the duration of acute pain (Chen et al., 2014 Cochrane Review) • For many people these medications are either partially or totally ineffective, whether administered alone or in combination • Pain management of complex and usually inadequate • Cochrane reviews have concluded that corticosteriods and acyclovir are ineffective at reducing the incidence of PHN, and there is insufficient evidence to determine whether other anti-virals are effective (Han et al., 2013 and Chen et al., 2014 Cochrane Review) What causes the pain during shingles and post-herpetic neuralgia? • • • • • The cause of the nerve pain is unknown It is thought that when VZV reactivates from neurons, the virus causes damage to these cells and this may play a role in pain The paucity of samples of human ganglia from donors during or following documented herpes zoster have significantly hampered the study of host immune responses to VZV in human ganglia We have sourced human ganglia from patients who at the time of death had a herpes zoster rash, but who had not died from VZV related causes Rather than direct virus induced damage, we showed that the damage to neurons is more probably due to our own immune system attacking and damaging infected neurons (Hood et al., 2006, Gowrishankar et al., 2010) Immune cells infiltrating the site of VZV infection Nerve cells (neurons) CD3+ T cells infiltrate into ganglia during active herpes zoster Herpes Zoster Ganglia 1 Herpes Zoster Ganglia 1 CD3+ stain S100 stain DAPI stain CD4+ stain S100 stain DAPI stain During active herpes zoster there is a robust infiltration of T cells into the affected ganglia, with CD4 cells predominating over CD8 cells Cytolytic nature of T cells during active herpes zoster CD8 Granzyme B A B C CD4 Granzyme B D Merge E Merge F Cytolytic CD8+ T cells are a prominent feature in ganglia during active herpes zoster CD8+ T cells interact with neurons-satellite glial cell units during active herpes zoster CD3 S100 B A CD8 D Merge C NCAM E Merge F Steain et al. Journal of Virology (2014) Histological examination of PHN-affected ganglia (>12 months post rash) Immunohistochemical examination of this tissue for immune cell subsets revealed CD3+ T cells and B cells are found to persist in PHN affected ganglia many months following herpes zoster rash resolution Immune response in human ganglia during or after herpes zoster • During active herpes zoster there is a robust infiltration of T cells into the affected ganglia, with CD4 cells predominating over CD8 cells • These infiltrating cells express markers of cytolytic potential • Cytolytic T cells closely associate with neuron-SCG units. This suggests that the host immune response may be the cause of damage to neuronal cells during herpes zoster, which then results in persisting pain • First detection of immune cell subsets within the ganglia of a PHN-affected patient • Demonstrates an ongoing immunological process >12 months after rash resolution in a patient suffering from PHN A vaccine to prevent chickenpox or shingles? • There are vaccines against chickenpox and against shingles • These vaccines are comprised of live attenuated VZV Chickenpox vaccine “Varivax” Shingles vaccine ‘Zostavax” 14x more concentrated • The molecular basis for this attenuation is not understood • These vaccines are regarded as “first generation” vaccines • Chickenpox vaccine is regarded as being highly immunogenic, efficacious and safe in preventing varicella (chickenpox) A vaccine to prevent chickenpox or shingles? • Chickenpox vaccine can still cause mild chickenpox • The shingles vaccine is only ~50% effective at stopping shingles (Oxman et al., NEJM) • Limitations of vaccines include: • Vaccinees can experience breakthrough infection • Unsuitable for use in the immunocompromised or pregnant individuals • Vaccine virus retains the ability to infect and spread to ganglionic neuronal and establish latency • Vaccine virus has been reported to reactivate in some people to cause to shingles and neuropathic pain Can we develop a better a “second” generation vaccine ? • A better (ie “second generation”) live attenuated vaccine virus would be incapable of establishing latency in neurons (ie lack neurotropism) • There has been at least one VZV neurotropic gene identified, and there are probably more. These need to be defined and deleted from the vaccine virus. Can we develop a better a “second” generation vaccine ? • VZV encodes multiple strategies to delaying and/or modulate the host immune response in a manner that is likely to confer a survival advantage to the virus • Examples include: • Downregulate cell-surface MHC class I expression • Modulates cell-surface MHC class II expression • Impairs dendritic cell (DC) functions • Inhibits interferon (IFN) responses • Identification of VZV encoded immunomodulatory genes and their removal from the vaccine virus may enhance immunogenicity of this vaccine Summary and Implications • Currently: • • • Large health care cost Success of anti-viral therapies only partial Vaccine should be improved • Better understanding of VZV pathogenesis • Prevention/ treatment/ management strategies for VZV induced diseases Acknowledgments VZV Lab members A/Prof Barry Slobedman (University of Sydney) Prof Tony Cunningham (Westmead Millennium Institute) A/Prof Michael Buckland (University of Sydney) Dr Michael Rodriguez (Dept of Forensic Medicine, NSW Pathology) Prof Ann Arvin (Stanford University) NHMRC Project grant funding



























![Antivirals are sometimes used. [46] [47]](http://s1.studyres.com/store/data/001011126_1-55d72bad7d922af991bf9fcdd0a95bd9-150x150.png)