* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Nitro drugs and the trypanosomatids
Polysubstance dependence wikipedia , lookup
Drug discovery wikipedia , lookup
Psychopharmacology wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Orphan drug wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug interaction wikipedia , lookup
Neuropharmacology wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
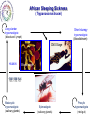
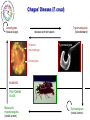
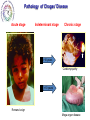
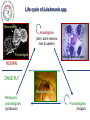
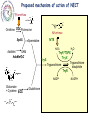
Parasitology 2017 Nitro drugs and the trypanosomatids Susan Wyllie ([email protected] Neglected Tropical Diseases: What are they? Disease (millions) (million DALYs)a (per annum) 35 89 1.5 million Tuberculosis 2,000 36 1.5 million Malariac 198 82.7 0.6 million Sleeping sickness Chagas' disease Leishmaniasis 0.4 8.0 12 1.6 0.6 2.0 10,000 13,000 30,000 Schistosomiasis Onchocerciasis Filariasis 200 18 120 1.8 1.0 5.6 15,000 0 0 BACTERIAL PROTOZOAL a Disability-adjusted Health burden Deaths HIV / AIDSb VIRAL HELMINTHIC Infected life years lost to the community, c.f. War = 20 million DALYs b 80% of all deaths in Africa c 90% of all deaths in Africa * World Health Reports, 2014 African Sleeping Sickness (Trypanosoma brucei) Long slender trypomastigote (blood and lymph) Short stumpy trypomastigote (bloodstream) CNS Stage HUMAN Blood stage TSE TSE FLY Metacyclic trypomastigote (salivary glands) Epimastigote (salivary glands) Procylic trypomastigote (mid-gut) Human African Trypanosomiasis Early stage Late stage CNS invasion Few weeksa to few monthsb aTrypanosoma brucei rhodesiense (10% of cases) bTrypanosoma brucei gambiense (90% of cases) Current Sleeping Sickness Drugs Suramin (1916) Pentamidine (1937) Melarsoprol (1946) Eflornithine (1977) (plus Nifurtimox 2009) Inactive in all late stage disease Parenteral administration (i.v.) Prolonged treatment (3 weeks) Toxicity (fatal anaphylaxis ~ 1 in 20,000; skin reactions, reversible renal damage) Future availability (Bayer) and cost Inactive in all late stage disease Parenteral administration (i.m. for 10 days) Inactive in some T.b.rhodesiense cases Toxicity (myalgia, diabetes, sterile abscess) Cost ($ 60-150) Severe toxicity (death 5% of patients) Parenteral administration (slow i.v. infusion) High relapse rate (drug resistance?) Prolonged hospitalisation (> 1 month) Future availability (Aventis) and cost Inactive against T.b.rhodesiense late stage Parenteral administration (i.v. infusions) Prolonged treatment (4 x / day for 2 weeks) Reversible toxicity (convulsions; bone marrow suppression; GI symptoms; nerve deafness) Future availability (Aventis) and cost ($700) Chagas’ Disease (T. cruzi) Amastigotes (tissue stage) Trypomastigotes (bloodstream) release and reinvasion Infected macrophage Trypomastigote Amastigote HUMANS TRIATOMINE BUGS Metacyclic trypomastigotes (rectal lumen) Epimastigotes (rectal lumen) Pathology of Chagas’ Disease Acute stage Indeterminant stage Chronic stage >10 years Cardiomyopathy Normal >10 years Romana’s sign Mega-organ disease Treatment of Chagas’ Disease Acute stage Chronic stage O O2N O N N S O H3C Nifurtimox (1964) NO2 N H N N O Benznidazole (1972) None Life cycle of Leishmania spp. Macrophage Amastigotes (skin, bone marrow liver & spleen) Promastigote Infected macrophages HUMAN SAND FLY Phlebotomus spp Metacyclic promastigotes (proboscis) Promastigotes (midgut) Pathology of the Leishmaniases CUTANEOUS MUCOCUTANEOUS VISCERAL L.major L.mexicana L.tropica L.braziliensis L.donovani L.infantum Drugs for Leishmaniasis Pentostam (SAG) Pentamidine Liposomal Amphotericin B (AmBisome) Drug resistance Parenteral administration Prolonged treatment (up to 4 weeks) Toxicity (liver and pancreas, HIV patients) Compliance / Cost ($ 120-150) HCOH HCO OH O- OCH HCO Sb O Sb OCH HCO + 3Na OCH COO- Parenteral administration (i.m.) Poor response rates (around 75%) Prolonged treatment (up to 4 weeks) Toxicity (e.g. myalgia, diabetes, sterile abscess) Cost ($ 60-150) Parenteral administration (slow iv infusion) Severe toxicity (e.g. renal failure, cardiotoxicity) Cost (AmBisome > $1000) Prolonged hospitalisation CH2OH CH2OH HCOH COO- NH NH H2N NH2 O O OH H3C HO OH O O CH3 OH OH OH OH Miltefosine Parenteral administration (i.m.) Prolonged treatment (up to 3 weeks) Toxicity (nephrotoxicity; nerve deafness) Availability / Cost ($50) Toxicity (teratogenic, pregnancy) Ease of resistance (long half life of drug) Compliance / Cost ($50) O H H3C O OH O HO HO NH2 O O H2 N H 2N O HO HO HO O H2N OH Paromomycin (Aminosidine) OH NH2 OH O NH2 O OH O H3C (CH2)14 O P O O- N+(CH)3 CH3 OH The Need for New Drugs - Summary Limitations of current anti-parasitic drugs • Toxicity (melarsoprol 5% deaths in HAT) • Teratogenicity (miltefosine for leishmaniasis) • Drug resistance (pentostam for leishmaniasis) • Efficacy (nifurtimox for Chagas’ disease) • Route of administration (all HAT drugs are by injection) • High cost (Ambisome >$1,000, Eflornithine ~$700) • Availability (eflornithine, melarsoprol) • Compliance (antimalarials) • Policy (artemesinin combination therapy) Pharmaceutical industry & unmet medical need • 17 new drugs developed for all tropical diseases 1975-2004 • Most inappropriate for resource poor settings • Diseases of the poor • Cost to develop new drug ~$800m (no market) The Ideal Target Product Profile for NTDs Safe for use: men, women, children and foetus Minimal toxicity: tolerable side effects; better than current drugs Few contraindications: drug-drug interactions; HIV or TB co-infections Efficacy: better than current drugs Compliance: short treatment; once per day Resistance: low potential to generate drug resistance Orally active: avoid needles and hospitalization Broad spectrum: all disease-causing species, including resistant lines Stable: 2 years shelf life at 40C and 75% relative humidity Affordable: diseases of poverty (cheaper than existing drugs) Nitro-heterocyclic Drugs Compounds which contain a nitro group attached to an aromatic ring system Therapeutics for a number of indications, particularly infectious diseases Metronidazole Antibiotic Antiprotozoal Nitrofurantoin Antibiotic (UTIs) Azathioprine Immunosuppressive Tolcapone Parkinson’s disease Chloramphenicol Antibiotic Flutamide Prostate cancer Nifedipine Angina Hypertension Nitro-heterocyclics in the treatment of infectious disease – the future Renewed interest in the use of nitroheterocyclics for the treatment of infectious disease PA-824 – phase II clinical trials against tuberculosis Nitazoxanide – used in the treatment of giardiasis, cryptosporidiosis and is in clinical trials for viral hepatitis Nitro-drugs and Chagas’ disease Nifurtimox and benznidazole have been used in the treatment of Chagas’ disease since the 1970’s Estimated global population infected with T. cruzi, 2009 * Nfx nitrofuran Bnz nitroimidazole Efficacy of Nfx and Bnz ~70% against acute stage <20% against chronic stage Low efficacy attributed to naturally occurring nitro-drug resistant strains (Filardi and Brener (1987) Trans. R. Soc. Trop. Med. Hyg. 81, 755-759.) Nitrodrugs against trypanosomiasis • Clinical trials of nifurtimox against HAT (1970s): • first clinical trial (Janssens and Demunynck (1977) – inconclusive, toxicity concerns – recommended only for melarsoprol-refractory patients • subsequent trials in the 1980s – variable efficacy (30 – 80%) – toxicity increasing with dose and treatment duration (30 – 60 days) – last resort for melarsoprol-refractory cases • clinical trials in 2001 – 2009: – three drug combinations - nifurtimox:eflornithine:melarsoprol (Priotto et al. (2006) PLoS Clin. Trials 1, e39) - (nifurtimox plus eflornithine, NECT) was found to be safer than (melarsoprol plus nifurtimox or plus eflornithine) – NECT is as effective as eflornithine monotherapy Nifurtimox-eflornithine combination therapy (NECT) for the treatment of HAT Eflornithine (1981) NECT (2009) Regimen 4 x daily for 14 days (56 slow IV infusions) IV 2 x daily for 7 days + oral Nfx 3 x 10 days Cost £407 / patient £203 / patient Side effects neuropenia anemia reduced Eflornithine (DFMO) Nifurtimox (Nfx) NECT • Nifurtimox-eflornithine combination therapy (NECT) added to the WHO list of essential medicines in 2009 Advantages of NECT • • • • • • • improved efficacy (>96%) reduced hospitalisation time reduced side effects reduced risk of infections reduced cost simplified logistics * reduced risk of drug resistance * NECT has become the treatment of choice for stage II T. b. gambiense HAT and is used to treat >90% of infections First new treatment for TriTryp diseases in >20 years Why do combination therapies protect against the emergence of drug resistance? Far easier for resistance to develop against a single drug (single mutation) than against a combination (chances of two advantageous mutations happening in one parasite exponentially higher) Drugs are often given in combination to protect against the development of resistance Drug combinations used to treat parasitic diseases include: Malaria: artesunate (oral artemisinin) should always be given in combination (mefloquine and amodiaquine often used) Malaria: Malarone contains a combination of atovaquone and proguanil Toxoplasmosis (in immuno-compromised patients): Pyrimethamine/sulfadiazine and folinic acid Proposed mechanism of action of NECT Targeting trypanothione metabolism Intracellular thiols COO- Monothiol O + H3N - COO N H SH H N + N H O O Dithiol OH O + H3N COO Mammals O H3N O Glutathione GSH H N - N H H N O SH SH H N O NH2+ O N H Trypanothione T[SH]2 Trypanosomatids Functions of Trypanothione Maintenance of thiol redox status • Reduction of protein and other disulphides (TryR) • Regulation of thiol redox potential (TryR and TryX) Defence against oxidant stress • Removal of peroxides via: 1.Type I tryparedoxin peroxidases (TryP) 2.Type II tryparedoxin peroxidases (TDPX) 3. Ascorbate peroxidase 4.Trypanothione S-transferase (eEF1B complex) Defence against chemical stress • Detoxification of methylglyoxal (glyoxalase I & II) • Xenobiotic metabolism (trypanothione S-transferase) Deoxyribonucleotide synthesis • Electron donor to ribonucleotide reductase via TryX TryR TryP TryX TDPX Glyoxalase 1 Proposed mechanism of action of NECT Eflornithine H2N F H2N F O OH Ornithine ODC Putrescine SpdS Spermidine N CH3 N N O OH NH2 O NH S COOH NH2 S O H3C Nifurtimox NTR NADP+ OH Glutamate + Cysteine GCS H3C N N H2O TryP/TDPX TryX TryS Trypanothione Trypanothione disulphide TryR NH2 N O H2O2 SAM AdoMet AdoMetDC N O O2N Glutathione NADPH NECT mechanism of action summary Eflornithine inhibits trypanothione biosynthesis – key in the parasite’s defence against oxidant stress Nifurtimox results in the generation of high levels of oxidant stress With impaired anti-oxidant defences (eflornithine), the parasite is far more susceptible to nifurtimox This is a great example of synergy between two drugs – work better in combination than individually Fexinidazole – the future Phase I clinical trials completed Phase II and III trials on-going in the Democratic Republic of Congo Proposed for use as the first orally available treatment for stage 1 and 2 HAT Nitro-drugs - key role in the future treatment of HAT Can fexinidazole also be an effective treatment for visceral leishmaniasis? In vivo metabolism of fexinidazole [O] [O] fexinidazole sulfoxide sulfone Fexinidazole is rapidly oxidised in vivo to sulfoxide and sulfone metabolites These metabolites accumulate to high levels in the blood following oral dosing of mice and likely to the therapeutically relevant species in vivo Sokolova et al., Antimicrobial Agents and Chemotherapy 54, 2893-2900 (2010) In vitro sensitivity of L. donovani to fexinidazole and its metabolites L. donovani EC50, µM Compound Structure Promastigote Amastigote (in macrophage) Fexinidazole sulfoxide 3.1 ± 0.3 5.3 ± 0.1 Fexinidazole sulfone 4.8 ± 0.1 5.3 ± 0.3 Miltefosine 6.1 ± 0.2 3.3 ± 0.2 5.6 ± 0.2 >50 Fexinidazole In vitro potency of fexinidazole sulfoxide and sulfone compares well with miltefosine 100 300,000 Suppression, % Parasite burden, LDU In vivo sensitivity of L. donovani to fexinidazole and its metabolites 200,000 100,000 0 75 50 ED50 – 12 mg kg-1 ED90 – 57 mg kg-1 25 0 50 100 on t C 150 Fexinidazole, mg kg -1 ro lv eh Pe ic le nt (0 os ) ta m M (1 ilt ef 5) os i ne Fe xi (1 ni 2) da z Fe ol e xi (2 ni 5) da zo Fe le xi ni (5 da 0) zo Fe le xi (1 ni 00 da ) zo le (2 00 ) 0 Drug treatment (mg kg -1) Fexinidazole proved to be an extremely effective, dose-dependent inhibitor Five single doses of 200 mg kg-1 suppressed infection by 99% ED50 estimated at 12 mg kg-1 At the lowest doses tested – fexinidazole equivalent to miltefosine 200 Pharmacokinetic properties of fexinidazole and its metabolites Blood concentration, ng ml-1 105 EC99 Sulfone 104 EC99 Sulfoxide sulfone sulfoxide 103 Oral dosing – 200 mg kg-1 102 fexinidazole 101 0 10 20 30 40 50 Time, h Blood levels of fexinidazole and major metabolites were determined by UPLC/MS/MS Fexinidazole has potential as a once daily, oral treatment for visceral leishmaniasis Fexinidazole: Determination of tissue distribution - Male albino rats dosed with [14C]-FEXINIDAZOLE - Tissue distribution determined by whole body autoradiography - Radioactivity distributed to all the body and was found in all organs and tissues analysed “Following oral dosing, highest radioactivity levels were measured in the intestinal wall, stomach, liver, kidney, prostate, pancreas, bladder, heart, muscle, spleen, thyroid and lung” Fexinidazole may be particularly suited for use in treatment of visceral leishmaniasis Torreele et al., PLoS Negl Trop Dis. 2010,4, e923 (2010) Cytocidal effect of fexinidazole sulfone on L. donovani 108 control Cell number, ml-1 7 10 106 + drug 105 104 103 0 10 20 30 Time, h Amastigotes incubated with 10x [EC50] fexinidazole sulfone At defined intervals, parasites were removed, washed and sub-cultured without drug Cells lost viability after 24h in the presence of drug Incredibly important that anti-trypanosomal drugs are cytotoxic The Ideal Target Product Profile for NTDs Safe for use: men, women, children and foetus Minimal toxicity: tolerable side effects; better than current drugs Few contraindications: drug-drug interactions; HIV or TB co-infections Efficacy: better than current drugs Compliance: short treatment; once per day √ √ Resistance: low potential to generate drug resistance Orally active: avoid needles and hospitalization √ Broad spectrum: all disease-causing species, including resistant lines Stable: 2 years shelf life at 40C and 75% relative humidity Affordable: diseases of poverty (cheaper than existing drugs) √ 34 √ Fexinidazole for VL - conclusions Fexinidazole has potential as a much needed additional oral therapy for visceral leishmaniasis Biological and pharmacokinetic properties of fexinidazole appear to be ideally suited for use against the severest form of leishmaniasis Comprehensive preclinical studies already completed on fexinidazole (phase I clinical trials for HAT) Every reason to hope that fexinidazole can progress rapidly into clinical development for use against visceral leishmaniasis Wyllie et al., Science Translational Medicine, 4, 119re1 (2012) Fexinidazole for visceral leishmaniasis DNDi undertook a Phase II proof of concept study to determine the efficacy and safety of fexinidazole in VL patients in 2013 Study was carried out in Sudan where current therapies (Amphoptericin B and Miltefosine are less effective) Advantages of trialling fexinidazole in Africa – effective treatments for VL are urgently needed for this region Outcome: 12 patients enrolled in the trial All demonstrated reduced parasite load during treatment although 9/12 relapsed following cessation of treatment Length of dose and general pharmacokinetics are to be further investigated Also, fexinidazole will be trialled as a partner drug for miltefosine (combination) Drugs for Neglected Diseases Initiative http://www.dndi.org/ WHO http://www.who.int/tdr/en/ [email protected] Medicines for Malaria Venture http://www.mmv.org/ Bill and Meilinda Gates Foundation http://www.gatesfoundation.org/ Reading list Nitro drugs for the treatment of trypanosomatid diseases: past, present, and future prospects. Patterson, S and Wyllie, S. Trends Parasitol. (2014) 30, 289-298. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Priotto et al., Lancet. (2009) 374, 56-64.