* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Development of the Respiratory System
Survey
Document related concepts
Anatomical terminology wikipedia , lookup
Vascular remodelling in the embryo wikipedia , lookup
Anatomical terms of location wikipedia , lookup
Lymphatic system wikipedia , lookup
Prenatal development wikipedia , lookup
Drosophila embryogenesis wikipedia , lookup
Transcript
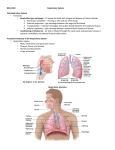
UNIVERSITY OF GUYANA MEDICAL REHABILITATION EMBRYOLOGY EMBRYOLOGICAL DEVELOPMENT OF: THE CARDIOVASCULAR SYSTEM & THE RESPIRATORY SYSTEM Group 1 Jennifer Haynes Anita Narine Neil Barry Zoe Daniels Origin/Derivative of Cardiovascular System Tissue of the cardiovascular system—heart, blood vessels, and blood Cells —originates from the mesodermal germ layer of the embryo. Although initially paired, by the 22nd day of development the two tubes form a single, slightly bent heart tube consisting of an inner endocardial tube and a surrounding myocardial mantle. During the 4th to 7th weeks the heart divides into a typical four-chambered structure. Development During the third week of gestation (days 15–21), the following stages occur: development of the primitive streak—gastrulation (day 15); formation of intraembryonic mesoderm (day 16); mesoblast differentiation—somatopleura and splanchnopleura (day 17); development of blood islets, the cardiogenic region, and primitive heart tubes (day 19); and formation of the primitive endocardial tube (day 21). During this time the bilaminar germ disc grows, especially in the cephalo-caudal axis. At the beginning of the third week, a midline structure called the primitive streakappears in the epiblast, near the caudal end of the disc. Epiblast cells detach along the primitive streak and migrate into the space between the epiblast and hypoblast layers as part of gastrulation. The migration forms a third layer called the intra-embryonic mesoderm (in red) (as seen in panel A) which goes to the cephalic end of the embryo. In this third layer, cellular groups, called blood islets, can be distinguished that form the shape of a horse shoe (panel B) . At the cranial end of the embryo, the blood islets form the cardiogenic region. Laterally, the angioblastic cords coalesce. On day 17, the lateral layer divides into two layers (panel C): the ventral layer will produce a pair of endocardial tubes and the dorsal layer will produce the two aortae (panel D). Embryonic folding brings the endocardial tubes into the ventral thorax where they fuse to form a single primitive heart tube(panel E). At this stage of development, the embryo is 2–3 mm long. Panel F shows the primitive heart tube. Starting at the inflow tract, we see the sinus venosus receiving the venous blood of the embryo, followed by the primitive atrium, the primitive ventricle, the bulbus cordis, and finally the truncus arteriosus which divides into paired dorsal aortae forming the outflow tract of the primitive heart tube. DEVELOPMENT OF SEPTUM The septum is the dividing wall between the right and left sides of the heart. That portion of the septum that separates the right and left atria of the heart is termed the atrial, or interatrial, septum, whereas the portion of the septum that lies between the right and left ventricles of the heart is called the ventricular, or interventricular, septum. Septum formation in the heart in part arises from development of endocardial cushion tissue in the atrioventricular canal (atrioventricular cushions) and in the conotruncal region (conotruncal swellings). Because of the key location of cushion tissue, many cardiac malformations are related to abnormal cushion morphogenesis. Septum Formation in the Atrium The septum primum, a sickle-shaped crest descending from the roof of the atrium, begins to divide the atrium in two but leaves a lumen, the ostium primum, for communication between the two sides. Later, when the ostium primum is obliterated by fusion of the septum primum with the endocardial cushions, the ostium secundum is formed by cell death that creates an opening in the septum primum. Finally, a septum secundum forms, but an interatrial opening, the oval foramen, persists. Only at birth, when pressure in the left atrium increases, do the two septa press against each other and close the communication between the two. Abnormalities in the atrial septum may vary from total absence to a small opening known as probe patency of the oval foramen. Septum Formation in the Atrioventricular Canal Four endocardial cushions surround the atrioventricular canal. Fusion of the opposing superior and inferior cushions divides the orifice into right and left atrioventricular canals. Cushion tissue then becomes fibrous and forms the mitral (bicuspid) valve on the left and the tricuspid valve on the right. Persistence of the common atrioventricular canal and abnormal division of the canal are well-known defects. Septum Formation in the Ventricles The interventricular septum consists of a thick muscular part and a thin membranous portion formed by (a)an inferior endocardial atrioventricular cushion, (b) the right conus swelling, and (c) the left conus swelling. In many cases these three components fail to fuse, resulting in an open interventricular foramen. Although this abnormality may be isolated, it is commonly combined with other compensatory defects. Septum Formation in the Bulbus. The bulbus is divided into (a) the truncus (aorta and pulmonary trunk), (b) the conus (outflow tract of the aorta and pulmonary trunk), and (c) the trabeculated portion of the right ventricle. The truncus region is divided by the spiral aorticopulmonary septum into the two main arteries. The conus swellings divide the outflow tracts of the aortic and pulmonary channels and with tissue from the inferior endocardial cushion close the interventricular foramen. Many vascular abnormalities, such as transposition of the great vessels and pulmonary valvular atresia, result from abnormal division of the conotruncal region; they may involve neural crest cells that contribute to septum formation in the conotruncal region. The aortic arches lie in each of the five pharyngeal arches. Four important derivatives of the original aortic arch system are (a) the carotid arteries (third arches); (b) the arch of the aorta (left fourth aortic arch); (c) the pulmonary artery (sixth aortic arch), which during fetal life is connected to the aorta through the ductus arteriosus; and (d) the right subclavian artery formed by the right fourth aortic arch, distal portion of the right dorsal aorta, and the seventh intersegmental artery. The most common vascular aortic arch abnormalities include (a) open ductus arteriosus and coarctation of the aorta and (b) persistent right aortic arch and abnormal right subclavian artery, both causing respiratory and swallowing complaints. The vitelline arteries initially supply the yolk sac but later form the celiac, superior mesenteric, and inferior mesenteric arteries, which supply the foregut, midgut, and hindgut regions, respectively. The paired umbilical arteries arise from the common iliac arteries. After birth the distal portions of these arteries are obliterated to form the medial umbilical ligaments, whereas the proximal portions persist as the internal iliac and vesicular arteries. Venous System Three systems can be recognized: (a) the vitelline system, which develops into the portal system; (b) the cardinal system, which forms the caval system; and (c) the umbilical system, which disappears after birth. The complicated caval system is characterized by many abnormalities, such as double inferior and superior vena cava and left superior vena cava. Lymphatic System The lymphatic system begins its development later than the cardiovascular system, not appearing until the fifth week of gestation. The lymphatic vessels originate from mesenchyme or may arise as saclike outgrowths from the endothelium of veins. Six primary lymph sacs are formed: two jugular, at the junction of the subclavian and anterior cardinal veins; two iliac, at the junction of the iliac and posterior cardinal veins; one retroperitoneal, near the root of the mesentery; and one cisterna chyli, dorsal to the retroperitoneal sac. Numerous channels connect the sacs with each other and drain lymph from the limbs, bodywall, head, and neck. Two main channels, the right and left thoracic ducts, join the jugular sacs with the cisterna chyli, and soon an anastomosis forms between these ducts. The thoracic duct then develops from the distal portion of the right thoracic duct, the anastomosis, and the cranial portion of the left thoracic duct. The right lymphatic duct is derived from the cranial portion of the right thoracic duct. Both ducts maintain their original connections with the venous system and empty into the junction of the internal jugular and subclavian veins. Numerous anastomoses produce many variations in the final form of the thoracic duct. PART II – RESPIRATORY SYSTEM When development does occur and description of the Respiratory System The respiratory system develops after the fourth week of gestation. The development of the respiratory system has many constituent components. Gaseous exchange occurs in the alveoli. The development of the respiratory system involves the endoderm and the mesoderm that surrounds it. The location of the bud along the gut tube is determined by signals from the surrounding mesenchyme, including fibroblast growth factors (FGFs) that “instruct” the endoderm. Hence epithelium of the internal lining of the larynx, trachea, and bronchi, as well as that of the lungs, is entirely of endodermal origin. The cartilaginous, muscular, and connective tissue components of the trachea and lungs are derived from splanchnic mesoderm surrounding the foregut Early Development The early development of the lungs lags behind the development of the heart and great vessels. However, as development proceeds the lungs will eventually occupy more of the thoracic cavity than the heart. This will be discussed further in the partitioning of the body cavities. Development of the Respiratory System We will begin with the development of the larynx and continue caudally. Larynx: The larynx is first seen as an outgrowth from the foregut. The outgrowth of tissue is called the respiratory diverticulum or the lung bud. The formation of the lung bud occurs when two lateral folds of splanchnic mesoderm and endoderm meet in the midline and separate the larynx and trachea from the esophagus. The lung bud is a ventral diverticulum of endoderm that arises from the floor of the foregut caudal to the pharynx. The diverticulum forms a groove in the floor of the pharynx called the laryngotracheal groove. Cephalic to the laryngotracheal groove is the epiglottal swelling. On either side of this groove are the developing arytenoid swellings. The epithelium of the larynx develops from the endoderm of the foregut. However, the muscles and cartilage arise from the 4th and the 6th arches. The development of these structures will be discussed in a later. Trachea: The trachea develops caudal to the larynx. The epithelium develops from the endoderm and the tracheal cartilage and muscles develop from splanchnic mesoderm. Early in development the trachea bifurcates into the left and right bronchi. Bronchi and Bronchioles: As the bronchi develop they continue to branch. The right bronchus gives off three diverticula and the left bronchus gives off two diverticula. These diverticula become the lobar bronchi and indicate that the right lung will have three lobes and the left lung will have two lobes. Each of the bronchi at this stage will divide into smaller bronchi. The branching of the bronchi continues until the bronchioles begin to form. In all there are 17 divisions of the bronchi until the sixth fetal month is reached. However, by early childhood there will be a total of 24 generations of branching that occurs. Maturation of the Lungs As the lungs develop and divide into smaller divisions there are changes in the vascular supply of the lungs as well. The lungs can be described as undergoing 4 phases of development. During the first phase of development, the pseudoglandular period, the bronchi are dividing into smaller and smaller units, the bronchioles. This period occurs from the 2nd month through the end of the 4th month. During the next 2 and 1/2 months the respiratory bronchioles are formed. They will give rise to alveolar ducts. This is called the canalicular period. During this time period the epithelium remains as a cuboidal epithelium and the capillaries while proliferating do not approach the respiratory epithelium. The next phase of development occurs from the 7th month until birth. During this period, the terminal sac phase / saccular phase, the number of capillaries increases and the capillaries approach the respiratory epithelium. At the same time the terminal sacs form. These result in the formation of a squamous epithelium, made up of type I alveolar epithelial cells, which will permit gaseous exchange. Hence, from the 7th month on the fetus is capable of survival. It is also starting with the 7th month that type II alveolar epithelial cells develop. These type II cells produce surfactant, the fluid that reduces the surface tension at the alveolar cell surface. Finally, from the 8th month on, the mature alveoli continue to be formed with an increase in the amount of surface area where capillaries and alveolar cells are in contact. This period of lung development is the alveolar period and actually can last through age ten. The growth of the lungs after birth is mainly the result of increases in the number of alveoli during this time. Table showing the Summary of Human Lung Stages/ Maturation of the Lungs Stage Embryonic Human Features week 4 to lung buds originate as an outgrowth from the ventral wall of 5 the foregut where lobar division occurs Pseudoglandu week 5 to conducting epithelial tubes surrounded by thick mesenchyme lar Canalicular Saccular Alveolar 17 week 16 to 25 week 24 to 40 are formed, extensive airway branching bronchioles are produced, increasing number of capillaries in close contact with cuboidal epithelium and the beginning of alveolar epithelium development alveolar ducts and air sacs are developed late fetal secondary septation occurs, marked increase of the number to 8 years and size of capillaries and alveoli Clinical Implications The absent or insufficient surfactant in the premature baby causes respiratory distress syndrome (RDS) because of collapse of the primitive alveoli (hyaline membrane disease). Congenital cysts of the lung; which are formed by dilation of terminal or larger bronchi. These cysts may be small and multiple, giving the lung a honeycomb appearance on radiograph, or they may be restricted to one or more larger ones. Cystic structures of the lung usually drain poorly and frequently cause chronic infections. The mesoderm surrounding the trachea inhibits branching whereas the mesoderm surrounding the bronchi stimulates branching. Formation of the respiratory system is regulated by a flow of molecules: Fibroblast growth factor 10 (FGF10) The Hox genes: Sonic hedgehog Other molecules in the mesoderm regulate branching and differentiation of epithelia of lung buds. The main regulatory molecules are: . N-Myc – stimulates branching Fibronectin and Collagen types I and III - stabilize branching sites Syndecan and Tenascin - stabilize the epithelia Epimorphin organizes the epithelium, including the polarity of the cells and their arrangement in the epithelium. Diagram showing asymmetric branching in week 5 forms the lobes of the lungs 3 secondary buds on the right bronchi; upper middle and lower lobe 2 secondary buds on the left bronchi; upper and lower lobe th In week 6 the 4 set of branching forms tertiary bronchi and the broncho-pulmonary segments; Ten on the right side and eight on the left side Right side Upper lobe – apical, anterior, posterior Middle lobe – medial, lateral Lower lobe – apical lateral basal , anterior basal , posterior basal Left side Upper lobe – apical, anterior, posterior Lower lobe – apical lateral basal , anterior basal , posterior basal During weeks 7 to 16 branching occur about 14 times to the level of terminal bronchioles. At this stage there are no alveoli. The fetal lung during this period is described as the glandular stage because the terminal bronchioles resemble glandular acini. The bronchi, containing cartilage in their walls, identify the sections as fetal lung. Development of the lung goes through 4 stages: The glandular stage – weeks 7 to 16 The canalicular stage – weeks 16 to 28 The saccular stage – weeks 28 to 36 The alveolar stage – weeks 36 to 40 Clinical Implications Babies born before 28 weeks have very small chances of survival because they lack pulmonary alveoli. A few manage to survive following intensive respiratory assistance. Babies born between 32 and 36 weeks usually suffer from the respiratory distress syndrome due to lack of surfactant secreted by pneumocytes type II. The respiratory distress syndrome is treated by: Intensive respiratory assistance . Surfactant therapy including surfactant lipoprotein and surfactant associated proteins A,B and C. REFERENCES o Langman's Medical Embryology 11th ed., Sadler, T W, (Thomas W.); Langman, Jan. Philadelphia : Wolters Kluwer Lippincott Williams & Wilkins o Developmental Biology, 6th ed., Gilbert, Scott F; Sunderland (MA): Sinauer Associates; 2000 o Larsen's human embryology 4th ed. Schoenwolf, Gary C; Larsen, William J, (William James). Philadelphia, PA : Elsevier/Churchill Livingstone http://search.medicinenet.com/search/search_results/default.aspx?Search what=1&query=mesoderm&I1=Search http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1767109/