* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download clostridium difficile associated diarrhea
Middle East respiratory syndrome wikipedia , lookup
Leptospirosis wikipedia , lookup
Oesophagostomum wikipedia , lookup
Carbapenem-resistant enterobacteriaceae wikipedia , lookup
Antibiotics wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Gastroenteritis wikipedia , lookup
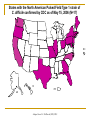
CLOSTRIDIUM DIFFICILE ASSOCIATED DIARRHEA VALERIE FLETCHER, M.D. INFECTIOUS DISEASES SOUTHERN OHIO MEDICAL CENTER August 2006 Introduction Clostridium difficile is a Gram-positive, spore-forming anaerobic bacillus. Most common cause of nosocomial diarrhea. Rate and severity of C. difficile-associated diarrhea (CDAD) increasing. New strain of C.difficile with increased resistance and virulence identified. History 1893 – first case of pseudomembraneous colitis reported as diphtheritic colitis. 1935 – “Bacillus difficile” isolated. 1970s – antibiotic-asociated colitis identified. 1978 – C. difficile toxins identified in humans. 1979 – therapy with vancomycin or metronidazole 2000 – increased incidence and virulence Annual CDAD rates for hospitals with >500 beds (NNIS 1987 – 2001) Estimates of US hospital discharges with CDAD listed as any diagnosis 70 Discharges per 100,000 60 50 40 30 20 10 0 1996 1997 1998 1999 2000 2001 Years Adapted from McDonald LC, et al. Annual Meeting of IDSA, 2005 2002 2003 Epidemiology Present in environment. Hospital is major reservoir. Spores can be recovered from surfaces for months. Spread primarily on hands of HCW. Fecal-oral transmission. Transmission may occur from asymptomatic colonized persons. Epidemiology Colonizes the colon of up to 3% of healthy adults. 15 – 25% of debilitated and antibiotic-treated hospitalized adults colonized. Toxigenic strains may cause disease in colonized patients. Implicated in approx. 25% of cases of antibioticassociated diarrhea Clinical features Mild disease – mild abdominal cramping pain. - endoscopic findings of diffuse or patchy, nonspecific colitis. Moderate disease – fever, dehydration, nausea, anorexia, malaise, profuse diarrhea, abdominal distention and cramping pain. - moderate leukocytosis, fecal leukocytes. - diffuse, patchy colitis on endoscopy Severe disease – usually profuse diarrhea, may be little or no diarrhea. - abdominal pain - fever - volume depletion - marked leukocytosis - peritoneal signs - radiologic signs include ileus, dilated colon and edematous colonic mucosa - endoscopic findings of adherent yellow plaques Complications of CDAD Pseudomembraneous colitis Toxic megacolon Perforation of the colon Sepsis Death Patients at increased risk for disease ANTIBIOTIC EXPOSURE Gastrointestinal surgery or manipulation Long length of stay in healthcare setting Infected roommate Co-morbid illnesses Immunosuppression Advanced age Proton-pump inhibitors and H2-blockers? Predictors of Severe Disease Leukocytosis > 20,000 Increased creatinine above the baseline Traditional list of Antibiotics associated with CDAD MORE FREQUENT LESS FREQUENT Cephalosporins (3rd and 4th generation) Ticarcillin-clavulanate Ampicillin/Amoxicillin Metronidazole Clindamycin Fluoroquinolones Other penicillins Rifampin Macrolides 5-Fluorouracil Tetracyclines Methotrexate Trimethoprim-Sulfamethoxazole Cyclophosphamide Laboratory Diagnosis Stool culture Latex agglutination to detect antigen in stools Tissue culture assay for cytotoxicity of toxin B Enzyme-linked immunosorbent assay (ELISA) for toxins A and B A new strain of C. difficile (NAP-1) Toxinotype III Unsuppressed production of toxins A and B Associated with presence of binary toxin. Increased resistance to clindamycin and fluoroquinolones. Potential for increased complications and adverse outcome. States with the North American Pulsed Field Type 1 strain of C. difficile confirmed by CDC as of May 15, 2006 (N=17) DC HI PR AK Adapted from L.C. McDonald, MD, CDC Rate of antibiotic resistance in 91 C. difficile isolates from a Pittsburg hospital that experienced a large CDAD outbreak beginning January 2000 Resistance (%) 100 90 80 70 60 50 40 30 20 10 0 Vancomycin Metronidazole Clindamycin Muto CA, et al. Infect Control Hosp Epidemiol 2005;26:273-280 Levofloxacin The risk of CDAD and antibiotic use in a study of 1,703 patients from 12 Canadian hospitals 4 3.5 Odds Ratio 3 2.5 2 1.5 1 0.5 0 Cephalosporins Fluoroquinolones Clindamycin Loo VG, et al. NEJM 2005;353:2442-2449. Macrolides Number of inpatients tested and percentage of stools positive for Clostridium difficile toxin at SOMC 2004 - 2006 300 Number of patients 250 200 150 100 50 0 J- F- M- A- M- J- J- A- S- O- N- D- J- F- M- A- M- J- J- A- S- O- N- D- J- F- M- A- M- J04 04 04 04 04 04 04 04 04 04 04 04 05 05 05 05 05 05 05 05 05 05 05 05 06 06 06 06 06 06 Time Inpatient tested no. Inpatient pos % Linear (Inpatient tested no.) Dr. T. Cassity, SOMC Linear (Inpatient pos %) Number of positive Clostridium difficile toxin tests SOMC laboratory 2003 -2006 40 Number of positive tests 35 30 25 20 15 10 5 0 J- F- M- A- M- J- J- A- S- O- N- D- J- F- M- A- M- J- J- A- S- O- N- D- J- F- M- A- M- J- J- A- S- O- N- D- J- F- M- A- M- J03 03 03 03 03 03 03 03 03 03 03 03 04 04 04 04 04 04 04 04 04 04 04 04 05 05 05 05 05 05 05 05 05 05 05 05 06 06 06 06 06 06 TIME Inpatient Outpatients Linear (Outpatients) Dr. T. Cassity, SOMC Linear (Inpatient) CDAD - SOMC 2006 114 patients had C. difficile toxin detected in stools between Jan 2006 – June 2006. 46 charts were available for review. 31 (67%) patients were female. 2 (4%) patients had recurrent CDAD. 13 (28%) patients did not receive a fluoroquinolone during or immediately before admission. Data from Marcie Malone, PharmD candidate Antibiotics associated with CDAD - SOMC 2006 Amp/Sulb Clindamycin Aztreonam Tobromycin Gentamicin Linezolid Ceftazidime Azithromycin Imipenem Ertapenem Cefazolin Cefepime Pip/Tazo Ceftriaxone Levofloxacin 0 5 10 15 20 25 Number of patients with positive C.difficile toxin Data from Marcie Malone, PharmD candidate 30 35 Ceftriaxone and Levofloxacin use – SOMC, Jan – Aug 2006 9000 Number of patients 8000 7000 6000 5000 4000 3000 2000 1000 0 Ceftriaxone IV Levofloxacin Oral Levofloxacin Antibiotics dispensed Proton Pump Inhibitors, H2-blockers and C.difficile toxin detection – SOMC 2006 Famotidine Lansoprazole Esomeprazole 0 5 10 15 20 Number of patients with positive C.difficle toxin Data from Marcie Malone. PharmD candidate 25 Management Enhanced infection control measures. Targeted antibiotic restriction Appropriate antibiotic therapy Adjunctive therapy – probiotics, IVIG, toxin binders Surgery Avoid antiperistaltic and opiate drugs. Experimental therapy – rifaximin, tolevamar, corticosteroids, vaccine, monoclonal antibodies to toxins A and B, non-toxigenic C,difficile Antibiotic Therapy Oral therapy – vancomycin, metronidazole Unable to tolerate oral therapy – IV metronidazole, vancomycin via NG tube or enema. Vancomycin + rifampin Less frequently used – Bacitracin, fusidic acid Antibiotic therapy for CDAD Metronidazole Vancomycin Route Oral or IV Oral only Dose 250 – 500 mg q8h or q6h 125 – 500 mg q6h Response rate 94% 94% Duration 10 – 14 days 10 – 14 days Cost $20 $200 - $800 Recurrence rate 20% 19% Indications for Vancomycin therapy No response to metronidazole Metronidazole intolerance Pregnancy and child < 10 yrs Severe/fulminant CDAD Conclusion Increasing numbers and severity of CDAD. Active surveillance recommended. Early diagnosis and treatment are important for reducing severe outcome. Judicious use of antibiotics may reduce incidence of CDAD Strict infection control practices essential.