* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Suppression of laser-induced choroidal neovascularization
Survey
Document related concepts
Transcript
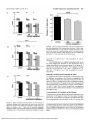
Suppression of Laser-Induced Choroidal Neovascularization by Oral Tranilast in the Rat 1,2 Yasuo Takehana, Torn Kurokawa, Tsuyoshi Kitamura, Yoshimi Tsukabara, Satoshi Akahane,1 Makio Kitazawa,1 and Nagahisa Yoshimura2 PURPOSE. TO determine whether tranilast administered to pigmented rats inhibits formation of choroidal neovascularization induced by diode-laser photocoagulation. Female Brown Norway rats were used. On day 0, choroidal neovascularization was induced by diode-laser photocoagulation, using a setting of 75 jum spot size, 0.1 second's duration, and 100 mW intensity. Tranilast (200 or 600 mg/kg per day) was administered orally twice daily for 14 days. Indomethacin (1 and 5 mg/kg per day) was administered orally once a day for 14 days. Choroidal neovascularization was evaluated on days 7 and 14 by fundus photography and fluorescein angiography. Late-phase fluorescein angiography was scored according to four grades. The animals were killed on day 14, and the lesions were evaluated histologically. METHODS. In the vehicle-treated group, 34 of 35 burns (97%) showed fluorescein staining and late leakage on day 14. Choroidal neovascularization was identified by light microscopy in all the lesions that showed fluorescein staining and late leakage. The score of fluorescein staining was reduced in rats given 200 mg/kg per day or 600 mg/kg per day (P < 0.01) of tranilast. The thickness of the laser-induced lesions was reduced in a dose-dependent manner by tranilast, a significant difference was observed with 600 mg/kg per day (P < 0.05). Oral indomethacin treatment did not reduce fluorescein staining on day 14. RESULTS. Tranilast inhibits the development of choroidal neovascularization in this experimental model. (Invest Ophthalmol Vis Sci. 1999;40:459-466) CONCLUSIONS. A ge-related macular degeneration (AMD) is the main cause of blindness among the elderly in developed countries.1"3 This condition is subdivided into exudative and dry forms. In exudative AMD, choroidal neovascularization (CNV) extends through Bruch's membrane and into the subretinal space, subretinal pigment epithelium, or both.6'7 The choroidal neovascular membrane then destroys the macula, which results in visual loss. Although submacular surgery to remove CNV has been advocated, the efficacy of this new therapy has yet to be convincingly demonstrated,8"11 and at present laser photocoagulation is the only well-established treatment. Unfortunately, however, the outcome of laser photocoagulation is not at all satisfactory. The Macular Photocoagulation Study Group12 reported that laser treatment of subfoveal CNV induced an immediate decrease in visual acuity, and they also showed that the neovascularization recurrence rate was rather high.13"15 Recently, antiangiogenic agents such as interferon16 and thalidomide17 have been used to suppress the development of neovascular membranes. However, systemic interferon-a has been shown to produce no real benefit in patients with neo- From the "Discovery Research Laboratory, R&D, Kissei Pharmaceutical Co., Minamiazumi; the 2 Department of Ophthalmology, Shinshu University School of Medicine, Matsumoto; and the 'Toxicology Laboratory, R&D, Kissei Pharmaceutical Co., Minamiazumi, Japan. Submitted for publication January 27, 1998; revised August 7, 1998; accepted September 21, 1998. Proprietary interest category: P. Reprint requests: Nagahisa Yoshimura, Department of Ophthalmology, Shinshu University School of Medicine, Matsumoto 390-8621, Japan. vascular AMD.18 The Age-Related Macular Degeneration and Thalidomide Study is evaluating the potential of thalidomide as an adjunctive treatment for patients with choroidal neovascular membranes associated with AMD. Recently, however, it was reported that thalidomide did not prevent the recurrence of a choroidal neovascular membrane in a patient with punctate inner choroidopathy.19 Tranilast is an antiallergic drug whose antiallergic effect is thought to result from inhibition of the release of chemical mediators from mast cells and basophils.20'21 Several studies have also shown that tranilast prevents or reduces the formation of keloids and hypertrophic scars through its inhibition of abnormal fibroblast proliferation, collagen synthesis, and the production of cytokines and oxygen-free radicals from activated macrophages and neutrophils.22"24 Furthermore, it has been reported that tranilast inhibits the proliferation and migration of smooth muscle cells and collagen synthesis by these cells.25 We have recently shown that tranilast inhibits some steps in the process of angiogenesis, including chemotaxis, proliferation, and tube formation of microvascular endothelial cells in vitro, and that it exerts an inhibitory effect on angiogenesis in vivo.26 In the present study, we examined the effect of the systemic administration, of-tranilast on experimental CNV created by diode-laser irradiation in pigmented rats. MATERIALS AND METHODS Experimental CNV Female Brown Norway (BN) rats (CLEA Japan, Tokyo, Japan) weighing between 220 and 300 g were used. All animal exper- Investigative Ophthalmology & Visual Science, February 1999, Vol. 40, No. 2 Copyright © Association for Research in Vision and Ophthalmology Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933430/ on 05/10/2017 459 460 Takehana et al. iments conformed to the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research. Rats were anesthetized with intraperitoneal pentobarbital (50 mg/kg), and the pupil was dilated by topical phenylephrine and tropicamide. Diode-laser photocoagulation was carried out using a diodelaser photocoagulator (OcuLight Six; IRIS Medical, Mountain View, CA) and a slit lamp delivery system with a Nikon slitlamp (model NS-1; Nikon, Tokyo, Japan). A handheld coverslip served as a contact lens. One or two burns were created between the major retinal vessels in each eye, using a setting of 75-/Am spot size, 0.1-second duration, and 100-mW intensity, as described previously.27 Evaluation of Time Course of Experimental CNV Five BN rats were used to establish the natural time course of diode-laser-induced CNV. On day 0, laser photocoagulation was performed as described above. The coagulated lesions (n = 20) were assessed on days 4, 7, 14, 21, 28, 56, and 301 by means of ophthalmoscopy, fundus photography, and fluorescein angiography. For fluorescein angiography, 1 ml/kg of 10% sodium fluorescein (Fluorescite; Alcon, Fort Worth, TX) was injected into the tail vein of anesthetized rats, and angiograms were taken using a Kowa fundus camera (GENESIS; Kowa, Tokyo, Japan). Evaluation of the Effects of Tranilast and Indomethacin on Experimental CNV A total of 96 female BN rats were divided into six groups. All rats underwent laser photocoagulation as described above. Group A rats (n = 19) received 100 mg/kg tranilast by mouth twice daily (total dose, 200 mg/kg per day) for 14 days. Drug administration was started immediately after photocoagulation (day 0) and continued until day 13- Group B rats (n = 14) were given 300 mg/kg tranilast twice daily (total dose, 600 mg/kg per day) for 14 days using the same schedule as for group A. Group C (n = 18) rats were used as controls for groups A and B and received 0.5% carboxymethylcellulose (CMC) by mouth instead of tranilast twice daily for 14 days. Group D rats (n = 15) received 1 mg/kg indomethacin orally once a day for 14 days. Drug administration was started immediately after photocoagulation (day 0) and continued until day 13. Group E rats (n = 14) were given 5 mg/kg indomethacin once a day for 14 clays using the same schedule as for group D. Group F (n = 16) rats were used as the control for groups D and E and received 0.5% CMC instead of indomethacin once a day for 14 days. Tranilast, vV-(3,4-dimethoxycinnamoyl) anthranilic acid, was synthesized in our laboratory and suspended in 0.5% CMC. Indomethacin was purchased from Merck (Rahway, NJ) and was suspended in 0.5% CMC. The lesions were studied on days 7 and 14 using ophthalmoscopy, fundus photography, and fluorescein angiography. Histopathologic Studies Fourteen days after photocoagulation, the rats were killed with an overdose of sodium pentobarbital. Eyes were enucleated and fixed overnight in 4% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, at 4°C. For light microscopy, tissue samples were dehydrated and embedded in paraffin. Then, serial sections were cut at 3 /xm thickness. Each section was cut perpendicularly to the retina, as confirmed by the number of nuclei in the inner nuclear layer of the IOVS, February 1999, Vol. 40, No. 2 healthy retina. Serial sections were stained in an ABC, ABC sequence with hematoxylin and eosin, Masson's trichrome, or immunohistochemically with anti-von Willebrand (VW) factor. Immunohistochemical staining with anti-VW factor was performed according to the manufacturer's protocol using the EPOS method (DAKO, Carpinteria, CA). Briefly, sections were deparaffinized and then treated with 3% hydrogen peroxide to block endogenous peroxidase activity. After washing with Tris-HCl buffer (pH 7.4), the sections were incubated with a rabbit anti-human VW factor antibody (DAKO) for 45 minutes at room temperature, rinsed with Tris-HCl buffer, and then treated with true blue (Funakoshi, Kyoto, Japan) for 5 minutes at room temperature. For transmission electron microscopy, eyes were fixed as described above, postfixed in 1% osmium tetroxide, and dehydrated in ethanol. After substitution with propylene oxide, the specimens were embedded in Epoxy resin. Thin sections (1 /Ltm) were stained with 0.05% toluidine blue in 0.1 M phosphate buffer (pH 7.4). Ultrathin sections were post-stained with 2% uranyl acetate-lead citrate and then examined with a JEM-1200EX electron microscope OEOL, Tokyo, Japan). Measurement of the Thickness of Retinal Lesions A quantitative morphometric assessment of thickness of the retinal lesions was carried out using a computer-assisted image analysis system in a masked fashion. Microscopic images (Olympus BX50 microscope with a working magnification of X200) of hematoxylin and eosin-stained retinal sections were acquired via a charge-coupled device video camera (model MCD-350; Olympus, Tokyo, Japan), digitized by an image-grabber board, and analyzed using analysis software (MacScope, version 2.3; Mitani, Fukui, Japan). The distance between the disrupted retinal pigment epithelium and the top of the lesion was measured. For each retinal lesion, approximately 30 sections were examined, with the thickest value used for the evaluation. These measurements were made by two examiners in a masked fashion. Effects of Tranilast and Indomethacin on Fluorescein Intensity Rat serum was prepared from untreated BN rats killed with an overdose of sodium pentobarbital. One milliliter of serum was mixed in a glass tube with 10 /xl of 100 mg/ml sodium fluorescein solution and either 5 /xl dimethyl sulfoxide or dimethyl sulfoxide with various quantities of drugs. After incubation at room temperature for 5 minutes, the fluorescein intensity was measured in a fluorescence spectrophotometer (model F-2000; Hitachi, Tokyo, Japan) with an excitation wavelength of 490 nm and an emission wavelength of 580 nm. Measurement of Tranilast Concentration in Rat Plasma Five Sprague-Dawley rats (SLC, Shizuoka, Japan) were used to study the pharmacokinetics of tranilast. Blood samples were collected from each rat at 0.25, 0.5, 1, 2, 3, 4, and 12 hours after oral administration of 100 mg/kg tranilast. In addition, three BN rats from group B were killed with an overdose of sodium pentobarbital after fluorescein angiographic evaluation on day 14, and blood samples were collected. All blood samples were centrifuged to provide plasma. Methanol (2 ml) and internal standard solution (100 jLtg/ml of Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933430/ on 05/10/2017 Tranilast Suppresses Experimental CNV IOVS, February 1999, Vol. 40, No. 2 HOOC MeO MeO FIGURE 1. Chemical structure of tranilast. AT-cinnamoyl anthninilic acid in acetonitrile, 200 /xl) were added to the plasma samples (100 /xl) and mixed for 15 minutes. The samples were centrifuged at 3000 rpm for 10 minutes, and the supernatant was subsequently evaporated to dryness under nitrogen at 40°C. The extract was reconstituted in 50% acetonitrile (150 ^,1) and acetic acid (10 /u,l), and 50 /txl was applied to a high-performance liquid chromatography (HPLC) system. The HPLC system consisted of a liquid chromatograph (model L-4000; Hitachi) with an UV detector, and recording was carried out at a detection absorbance setting of 350 nm. The HPLC column (Develosil ODS-HG-5 [4.6 X 250 mm]; Nomura Chemical, Seto, Japan) was maintained at 50°C. The mobile phase consisted of 0.05% trifluoroacetic acid and acetonitrile (60:40), and it was pumped at a flow rate of 1.0 ml/min. 461 Evaluation and Statistical Analysis For the evaluation of drug treatment, the intensity of staining in late-phase (100-140 seconds after dye injection) fluorescein angiography was graded by two examiners in a masked fashion. Angiograms were graded as follows: Score 0, no staining; score 1, slightly stained; score 2, moderately stained; score 3, strongly stained (Fig. 2), and the score was evaluated by a Wilcoxon signed rank test. When the two scores given for a particular lesion did not coincide, the higher score was used for the analysis. Such discrepant scoring was observed in only 12 of the 171 lesions analyzed, and the discrepancy was never by more than one grade. The thickness of lesions was evaluated using sections from the central part of the lesion, which exhibited the thickest laser-induced retinal destruction. The thickness of the retinal lesions and the gain in body weight of the rats were evaluated by ANOVA followed by Dunnett's post hoc test. Values of P < 0.05 were considered statistically significant for all forms of statistical analysis used. Data are shown as means ± SEM. RESULTS Time Course of Changes in Staining Intensity of Fluorescein Angiograms after Photocoagulation The time course of changes in the staining intensity in fluorescein angiograms in nontreated rats was evaluated for up to 301 FIGURE 2. Representative late-phase fluorescein angiograms taken 14 days after diode-laser photocoagulation. Photocoagulation was carried out using a setting of 75-fJLm spot size, 0.1-second duration, and 100-mW intensity. One or two burns were created between the major retina! vessels in each eye. The intensity of fluorescein staining of each lesion in the late-phase angiograms was graded by two independent examiners. The angiograms were graded as follows: score 0, no staining; score 1, slightly stained; score 2, moderately stained; and score 3, strongly stained. Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933430/ on 05/10/2017 462 Takehana et al. IOVS, February 1999, Vol. 40, No. 2 inhibits the intensity of fluorescein staining in experimental CNV (Fig. 5C). Thickness of the Lesions The thickness of the lesions, which was evaluated morphometricaily in serial sections, was 48.7 ± 30 ju.ni (n ~ 33), 43.0 ± 2.9 ^m (n = 21), and 54.9 ± 3.5 /am (n = 32) in groups A, B, and C, respectively. Thus, lesions were less thick in tranilast-treated rats, although the effect was statistically significant only at 600 mg/kg per day (P < 0.05; Figs. 4 and 6). 2- 1- 4 7 14 21 28 56 300 Time after photocoagulation (day) FIGURE 3. Time course of changes in intensity of fluorescein staining in angiograms taken after diode-laser photocoagulation in nontranilasttreated rats. Each point indicates the mean of the scores of 9 to 20 lesions. Fluorescein staining was first observed 4 days after photocoagutation. Intensity of the staining then increased, and it reached its peak approximately 14 days after photocoagulation. Strong fluorescein staining was still present 301 days after treatment, the time of the last observation. days after photocoagulation. As shown in Figure 3, weak fluorescein staining was observed on day 4. Thereafter, the intensity increased, reaching its peak on about day 14. Strong fluorescein staining was still present on day 301. Histopathologic Study To determine the extent of the extracellular matrix, especially collagen, in the lesions, we used Masson's trichrome staining. Fourteen days after photocoagulation, collagen could be seen in laser-induced lesions by blue staining, and cells could be identified within the lesions by red staining (graphic data not shown). In terms of the intensity of collagen staining, there was no apparent difference between the lesions in the control and tranilast-treated groups. Electron microscopic evaluation revealed that new choroidal vessels were present within the lesions (graphic data not shown), but there was no apparent difference between the control and tranilast-treated groups. Body Weight Gain in Rats There was no significant difference between the control and tranilast-treated groups in the gain of body weight. The values were 27.1 ± 3.4g/l4d(n = 13), 19.2 ± 3.1 g/14 d (n = 11), Choroidal Neovascularization Because the intensity of fluorescein staining reached its peak on about day 14, we decided to evaluate the effect of tranilast on experimental CNY by fluorescein angiography on day 14. Hyperfluorescence was observed in almost all lesions in group C animals (control rats for groups A and B). On day 7, nearly all burns (31/32, 96.9%) showed fluorescein staining and late leakage; on day 14, 34 of 35 (97.1%) coagulated sites showed changes suggestive of CNV. Histologic assessment confirmed the presence of CNV in 100% of those lesions that were graded as score 1 or higher hyperfluorescence (Fig. 4). Immunostaining of sections with anti-VW factor antibody revealed stained endothelial cells within the lesion in all burns graded as score 1 or higher (graphic data not shown). Fluorescein Angiographic Evaluation of CNV On day 7, group A and group B rats showed a somewhat weaker staining intensity in fluorescein angiograms than did group C (control) rats. However, there was no statistically significant difference between the control group and the tranilast-treated groups (Fig. 5A). On day 14, the score given to the staining influoresceinangiograms was lower than control in both tranilast groups, the effect appeared to be dose-dependent (Fig. 5B). In fact, oral tranilast decreased the number of spots that showed strong dye staining (score 3) and increased the number showing weak staining (score 1). Group B (600 mg/kg tranilast per day) showed significantly lower scores than did the control group (P < 0.01). In contrast, there was no statistically significant difference between the control and indomethacin-treated groups, although there was noted a tendency that oral indomethacin ^ ^ FIGURE 4. light microscopy of retinal lesions from control (A) and tranilast-treated (B; 600 mg/kg per day) rats 14 days after diode-laser photocoagulation. Retinal sections were stained with hematoxylin and eosin. (A) A thin multikiyered fusiform membrane is seen in the central area of the lesion internal to the choroid. (B) Thin proliferative membranes are seen in a tranilast-treated rat. Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933430/ on 05/10/2017 IOVS, February 1999, Vol. 40, No. 2 Tranilast Suppresses Experimental CNV 463 oooo p<0.05 T oo oo control oo ooooo o 200 600 tranilast (mg/kg/day) p<0.01 control 200 600 tranilast (mg/kg/day) FIGURE 6. Effect of tranilast on thickness of the retinal lesions 14 days after photocoagulation. Each column shows the mean ± SEM of measurements taken from 21 to 33 sections. Tranilast reduced the thickness of the lesions in a dose-dependent manner. The lesions in the group treated with tranilast at 600 mg/kg per day were significantly less thick (P < 0.05) than those in the control group. B control 200 600 tranilast (mg/kg/day) ~p ooooo oooo and 24.0 ± 4.2 g/14 d (n = 12) in groups A, B, and C, respectively. Oral administration of 1 mg/kg indomethacin did not affect body weight gain. However, it was decreased in the 5 mg/kg indomethacin-treated group (group E). In group E, 7 animals died during the experiment. The changes in weight were 132 ± 2.0 g/14 d (n = 15), -0.8 ± 1.2 g/14 d (n = 7), and 10.2 ± 0.8 g/14 d (n = 16) in groups D, E, and F, respectively. Intensity of Fluorescein Staining In Vitro To confirm that the presence of tranilast or indomethacin does not influence the grading of the fluorescein angiograms, the effect of drugs on fluorescein intensity was assessed in vitro. As shown in Table 1, tranilast had no effect on fluorescein intensity at concentrations of 10 /xg/ml or less. With 100 /xg/ml and 1000 /xg/ml tranilast, the fluorescein intensity was increased to 1.5 and 1.7 times that of control, respectively. Indomethacin also had no effect onfluoresceinintensity at concentrations of 100 /xg/ml or less. Concentration of Tranilast in Rat Plasma control indomethacin (mg/kg/day) FIGURE 5. Effect of tranilast and indomethacin on the intensity of fluorescein staining. Each column shows the mean score; each circle shows the intensity score offluoresceinstaining in a single lesion. (A) Effects of tranilast at day 7. There was no statistically significant difference between the control and tranilast-treated groups. (B) Effects of tranilast at day 14. The intensity offluoresceinstaining was weaker As stated above, tranilast affected fluorescein intensity at concentrations of 100 jag/ml or more. For this reason, measurement of the concentration of tranilast in rat plasma after fluorescein angiography was performed on day 14, one day after in tranilast-treated rats than in control rats. Tranilast at 600 mg/kg per day showed significantly lower scores than did the control group (P < 0.01). (C) Effects of indomethacin at day 14. There was no statistically significant difference between the control and indomethacin-treated groups. Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933430/ on 05/10/2017 464 Takehana et al. IOVS, February 1999, Vol. 40, No. 2 1. Effect of Tranilast and Indomethacin on Fluorescein Intensity In Vitro TABLE Fluorescein Intensity (%) Tranilast 0.5% DMSO 0.1 /xg/ml 1 /xg/ml 10 jug/ml 100 /xg/ml 1000 ixe/ml 100 101.3 103.8 108.2 152 171.7 Indomethacin 100 105.2 104.3 102.0 163.2 DMSO, dimethyl sulfoxide. cessation of treatment with 600 mg/kg per day. The mean plasma concentration of tranilast was 0.87 /xg/ml (minimum, 0.11 /xg/ml; maximum, 2.26 /xg/ml; n = 3). As can be seen in Table 1, tranilast at these concentrations did not affect fluorescein intensity. Pharmacokinetics of Tranilast in Rat Plasma After oral administration of 100 mg/kg tranilast, the highest plasma concentration (Cmax), which was 130.9 M-g/ml, was obtained at 15 minutes, but this concentration then declined rather quietly; the half-life (t 1/2 ) was 4.5 hours (Fig. 7). Area under the curve (AUC) was 281.2 /xg-h/ml. DISCUSSION Choroidal neovascularization and its sequelae are major causes of visual loss. Hence, a new therapy for CNV in AMD has been long-awaited. Animal (monkey) models of laser-induced CNV were described nearly 20 years ago by Ryan28 and by Archer and Gardiner.29 Since then many animal models, including rabbits,30'31 cats,32'33 and rats,34"37 have been developed and used to evaluate the effect of various antiangiogenic agents on CNV. Of the numerous animal models, the pigmented rat has been used by several groups because its retina is similar to the human retina inasmuch as it is homogeneously pigmented and exhibits a high incidence of CNV after photocoagulation. Although the CNV induced by laser treatment may differ from that which occurs naturally in human eyes, this reproducible model is useful for both basic investigations of choroidal angiogenesis and for studies of its modulation by drug therapy. In the study described herein, we produced experimental CNV in the subretinal space of pigmented rats by diode-laser photocoagulation. Because no detailed study has been published on the time course of CNV after diode-laser photocoagulation in rats, we first studied this aspect of CNV. In our model, fluorescein-staining associated with CNV was first observed on day 4 (i.e., 4 days after photocoagulation), reached its peak on day 14, and was still at a high level on day 301 (Fig. 3). On the basis of these findings, we opted to evaluate the suppressive effect of tranilast on day 14. On day 14 after diode-laser photocoagulation, nearly all burns (34/35, 97%) showed fluorescein staining and late leakage suggestive of CNV. In contrast, using krypton laser photocoagulation in pigmented rats, Frank et al.38 reported that CNV occurred in only 6 of 13 lesions (46%), and Dobi et al.27 reported fluorescein angiographic leakage in only 24 of 86 lesions (28%) after 2 weeks. Wavelength absorption curves suggest that the transmission of diode-laser energy (780 - 850 nm) through the blood should be equal to or greater than that of krypton red irradiation.39 Therefore, superior transmission of diode-laser energy may be one of the reasons why we succeeded in producing highly reproducible CNV. In the present study, tranilast administered orally at 200 mg/kg per day and 600 mg/kg per day reduced the intensity of staining on fluorescein angiograms and the thickness of lesions in a dose-dependent manner in our experimental model of CNV (Figs. 5, 6). The dosages of tranilast used in this model are about the same as the effective dosage used in the experimental allergy,20 keloids and hypertrophic scars,22 and vascular restenosis studies40 in rats. The present pharmacokinetic data showed that the maximum plasma concentration was 130.9 /xg/ml after oral administration of tranilast at a dose of 100 mg/kg (Fig. 7). Recently, we reported that tranilast inhibits several important steps in angiogenesis-namely, proliferation, vascular endothelial growth factor-induced chemotaxis, and tube formation by human dermal microvascular endothelial cells-with IC50 values of 44.5 M-g/ml, 44.2 /xg/ml, and 57.3 ixg/ml, respectively.26 Moreover, at doses of 30 /xg/ml or more of tranilast inhibited proliferation and collagen synthesis by fibroblasts derived from human keloids and hypertrophic scars.23 Because a plasma concentration of more than 30 /xg/ml was maintained for only 2 hours with a single administration of 100 mg/kg tranilast (Fig. 7), twice daily administration of 100 mg/kg tranilast is the minimal dose in rats to obtain pharmacologically effective plasma concentrations of the drug. It has been reported that topical treatment with indomethacin suppressed corneal neovascularization via inhibition of prostaglandin production.41"44 Moreover, intravitreal administration of indomethacin is known to reduce the incidence of the subretinal neovascularization typically induced by argon laser photocoagulation in primates.45 However, oral administration of indomethacin did not inhibit CNV in the present study. It is possible that a sufficient concentration of indomethacin was not obtained in the choroid by the oral administra10001 100" o o 10 (0 CO m a. 2 4 6 8 10 12 14 Time after administration (hr) FIGURE 7. Plasma concentration of tranilast after a single orally administered dose of 100 mg/kg tranilast in SD rats. Each point represents the mean ± SEM (n = 5). Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933430/ on 05/10/2017 IOVS, February 1999, Vol. 40, No. 2 tion. Higher doses of indomethacin were toxic to the rats because their weight gain was markedly less than normal, and half of the animals died during the follow-up period. In contrast to indomethacin, tranilast does not affect the activity of cyclooxygenase in vitro,46 so the suppressive effects of tranilast on experimental CNV are not related to the inhibition of cyclooxygenase pathways. Tranilast has been used clinically in Japan in the treatment of asthma, allergic rhinitis, allergic conjunctivitis, keloids, and hypertrophic scars at a dose of 300 mg/d, and it was recently reported that tranilast reduced the rate of post-percutaneous transluminal coronary angioplasty restenosis at a dose of 600 mg/d in a phase III clinical trial.47 The doses of tranilast in clinical use are far less than those used in this rat experiment, so difference may be explained by different pharmacokinetics of tranilast between humans and rats. When tranilast (200 mg, single dose) was administered orally to patients with angina, the maximum plasma concentration was obtained at 4.7 hours, and Cmax was 31.7 /Ltg/ml. The t, /2 was 8.4 hours, and the AUC was 3692 jLtg-h/ml (Hideo Tamai, personal communication, 1997). On the basis of this result, using a nonlinear leastsquares regression computer program (WinNonlin version 1.1; Scientific Consulting, Cary, NC), we estimated the plasma concentration of tranilast when 200 mg of the drug was administered three times a day for 7 days in humans. The estimated maximum plasma concentration was 730 ju-g/ml. Using the pharmacokinetic data obtained in the present study (Cmax = 130.9 jag/ml, time to peak plasma concentration = 0.25 hour, t I/2 = 4.5 hours, AUC = 281.2 jLtg-h/ml), we also estimated the plasma concentration of tranilast when the drug was administered twice daily at 100 mg/kg for 7 days in the same manner: the estimated maximum plasma concentration of tranilast was 142.0 jLtg/ml. Therefore, the maximum plasma concentration and AUC in humans and rats are almost equivalent. In our assessment of intensity of fluorescein staining, it is important to note that the grading system is arbitrary and thus may not actually reflect a difference in CNV including that related to AMD. Moreover, tranilast did not extinguish fluorescein staining and leakage, rather it suppressed CNV as assessed by our grading system. Besides, with tranilast treatment, the number of eyes that had no leakage at day 14 was not different from that of the control group. There is a possibility that any leakage may indicate the lack of efficacy of suppressing laserinduced CNV. However, it is noteworthy that oral indomethacin did not suppress CNV according to the same grading system. It is also important to determine whether tranilast affects the intensity of fluorescein staining. Based on the in vitro effects of tranilast on fluorescein intensity and on the pharmacokinetic data (Table 1), it is possible that our grading of photocoagulation spots on day 7 may have been erroneously high (i.e., we may have underestimated the therapeutic effects of tranilast at this time point). On the other hand, medication was stopped the day before the fluorescein angiograms were obtained on day 14, and on day 14 the concentration of tranilast in plasma was mvich less than 100 jug/ml. On this basis, the effect of tranilast on the intensity of staining on the fluorescein angiograms would have been negligible, which is true also for indomethacin (Table 1). Although the data presented in this report show rather modest effects of tranilast on the inhibition of CNV, to the best of our knowledge, there are currently no known orally admin- Tranilast Suppresses Experimental CNV 465 istered drugs that suppress experimental CNV. Even though the effects of tranilast were modest in the present study, we believe it is worthwhile to study the clinical application of tranilast for human AMD, perhaps in combination with more effective drugs administered locally. References 1. Hyman LG, Lilienfeld AM, Ferris FL III, Fine SL. Senile macular degeneration: a case-control study. Am J Epidemiol. 1983; 118: 213-227. 2. Ferris FL III, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:l640-l642. 3. Goldberg J, Flowerdew G, Smith E, et al. Factors associated with age-related macular degeneration: an analysis of data from the first National Health and Nutrition Examination Survey. Am J Epidemiol. 1988;128:700-710. 4. The Eye Disease Case-Control Study Group. Risk factors for neovascular age-related macular degeneration. Arch Ophthalmol. 1992;110:1701-1708. 5. Klein R, Klein BEK, Unton KLP. Prevalence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1992; 99:933-943. 6. Sarks SH. New vessel formation beneath the retinal pigment epithelium in senile eyes. BrJ Ophthalmol. 1973;57:951-965. 7. Green WR, Key SN III. Senile macular degeneration: a histopathologic study. Trans Am Ophthalmol Soc. 1977;75:180-254. 8. Thomas MA, Grand MG, Williams DF, Lee CM, Pesin SR, Lowe MA. Surgical management of subfoveal choroidal neovascularization. Ophthalmology. 1992;99:952-968. 9- Bressler NM. Submacular surgery: are randomized trials necessary? Arch Ophthalmol. 1995; 113:1557-1560. 10. Bressler NM. Submacular surgery: new information, more questions. Arch Ophthalmol. 1997;115:1071-1072. 11. Kaplan H. Submacular surgery for choroidal neovascularization. Br J Ophthalmol. 1996;80:101. 12. Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 1991;109: 1220-1231. 13. Macular Photocoagulation Study Group. Recurrent choroidal neovascularization after argon laser photocoagulation for neovascular maculopathy. Arch Ophthalmol. 1986;104:503-512. 14. Macular Photocoagulation Study Group. Persistent and recurrent neovascularization after krypton laser photocoagulation for neovascular lesions of ocular histoplasmosis. Arch Ophthalmol. 1989; 107:344-352. 15. Macular Photocoagulation Study Group. Persistent and recurrent neovascularization after krypton laser photocoagulation for neovascular lesions of age-related macular degeneration. Arch Ophthalmol. 1990;108:825-831. 16. Brouty-Boye D, Zetter BR. Inhibition of cell motility by interferon. Science. 1980;208:5l6-518. 17. D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994;91:40824085. 18. Pharmacological Therapy for Macular Degeneration Study Group. Interferon alfa-2a is ineffective for patients with choroidal neovascularization secondary to age-related macular degeneration: results of a prospective randomized placebo-controlled clinical trial. Arch Ophthalmol. 1997; 115:865-872. 19. Ip M, Gorin MB. Recurrence of a choroidal neovascular membrane in a patient with punctate inner choroidopathy treated with daily doses of thalidomide. AmJ Ophthalmol. 1996; 122:594 -595. 20. Koda A, Nagai H, Watanabe S, Yanagihara Y, Sakamoto K. Inhibition of hypersensitivity reactions by a new drug, N-(3',4'-dimethoxycinnamoyl) anthranilic acid (N-5')- / Allergy Gin Immunol. 1976;57:396-407. 21. Komatsu H, Kojima M, Tsutsumi N, et al. Study of the mechanism of inhibitory action of tranilast on chemical mediator release. Jpn J Pharmacol. 1988;46:43-51. Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933430/ on 05/10/2017 466 Takehana et al. 22. Isaji M, Aruga N, Naito J, Miyata H. Inhibition by tranilast of collagen accumulation in hypersensitive granulomatous inflammation in vivo and of morphological changes and functions of fibroblasts in vitro. Life Sci. 1994;55:PL287-PL292. 23. Suzawa H, Kikuchi S, Arai N, Koda A. The mechanism involved in the inhibitory action of tranilast on collagen biosynthesis of keloid fibroblasts. Jpn J Pharmacol. 1992;60:91-96. 24. Suzawa H, Kikuchi S, Ichikawa K, Koda A. Inhibitory action of tranilast, an anti-allergic drug, on the release of cytokines and PGE2 from human monocytes-macrophages. Jpn J Pharmacol. 1992;60:85-90. 25. Tanaka K, Honda M, Kuramochi T, Morioka S. Prominent inhibitory effects of tranilast on migration and proliferation of and collagen synthesis by vascular smooth muscle cells. Atherosclerosis. 1994;107:179-185. 26. Isaji M, Miyata H, Ajisawa Y, Takehana Y, Yoshimura N. Tranilast inhibits the proliferation, chemotaxis and tube formation of human microvascular endothelial cells in vitro and angiogenesis in vivo. Br J Pharmacol. 1997;122:106l-1066. 27. Dobi ET, Puliafito CA, Destro M. A new model of experimental choroidal neovascularization in the rat. Arch Ophthalmol. 1989; 107:267-269. 28. Ryan SJ. The development of an experimental model of subretinal neovascularization in disciform macular degeneration. Trans Am Ophthalmol Soc. 1979;77:707-745. 29- Archer DB, Gardiner TA. Experimental subretinal neovascularization. Trans Ophthalmol Soc UK. 1980;100:363-368. 30. Van der Zypen E, Fankhauser F, Raess K. Choroidal reaction and vascular repair after chorioretinal photocoagulation with the freerunning neodymium-YAG laser. Arch Ophthalmol. 1985; 103:580589. 31. Van der Zypen E, Fankhauser F, Raess K, England C. Morphologic findings in the rabbit retina following irradiation with the freerunning neodymium-YAG laser: disruption of Bruch's membrane and its effect on the scarring process in the retina and choroid. Arch Ophthalmol. 1986; 104:1070-1077. 32. Perry DD, Risco JM. Choroidal microvascular repair after argon laser photocoagulation. Am] Ophthalmol. 1982;93:787-79333- Perry DD, Reddick RL, Risco JM. Choroidal microvascular repair after argon laser photocoagulation: ultrastructural observations. Invest Ophthalmol Vis Sci. 1984;25:1019-1026. 34. Pollack A, Heriot WJ, Henkind P. Cellular processes causing defects in Bruch's membrane following krypton laser photocoagulation. Ophthalmology. 1986;93:1113-1119. IOVS, February 1999, Vol. 40, No. 2 35. Pollack A, Korte GE, Weitzner AL, Henkind P. Ultrastructure of Bruch's membrane after krypton laser photocoagulation, I: breakdown of Bruch's membrane. Arch Ophthalmol. 1986;104:13721376. 36. Pollack A, Korte GE, Heriot WJ, Henkind P. Ultrastructure of Bruch's membrane after krypton laser photocoagulation, II: repair of Bruch's membrane and the role of macrophages. Arch Ophthalmol. 1986;104:1377-1382. 37. Baurmann H, Sasaki K, Chioralia G. Investigations on laser coagulated rat eyes by fluorescence angiography and microscopy. GraefesArch Clin Exp Ophthalmol. 1975; 193:245-252. 38. Frank RN, Das A, Weber ML. A model of subretinal neovascularization in the pigmented rat. Curr Eye Res. 1989;8:239-247. 39. Mainster MA. Wavelength selection in macular photocoagulation: tissue optics, thermal effects, and laser systems. Ophthalmology. 1986;93:952-958. 40. Kosuga K, Tamai H, Ueda K, et al. Effectiveness of tranilast on restenosis after directional coronary atherectomy. Am Heart J. 1997;134:712-718. 41. Thomas A, Deutsch AB, Hughes WF. Suppressive effects of indomethacin on thermally induced neovascularization of rabbit corneas. Am] Ophthalmol. 1979;87:536-540. 42. Harvey FT, Cherry PMH. Indomethacin v. dexamethasone in the suppression of corneal neovascularization. Can J Ophthalmol. 1983:18:293-295. 43. Frucht J, Zauberman H. Topical indomethacin effect on neovascularization of the cornea and on prostaglandin E2 levels. Br J Ophthalmol. 1984;68:656-659. 44. Haynes WL, Proia AD, Klintworth GK. Effect of inhibitors of arachidonic acid metabolism on corneal neovascularization in the rat. Invest Ophthalmol Vis Sci. 1989;30:1588-1593. 45. Sakamoto T, Soriano D, Nassaralla J, et al. Effect of intravitreal administration of indomethacin on experimental subretinal neovascularization in the subhuman primate. Arch Ophthalmol. 1995; 113:222-226. 46. Komatsu H, Kojima M, Tsutsumi N, et al. Mechanism of inhibitory action of tranilast on the release of slow reacting substance of anaphylaxis (SRS-A) in vitro: effect of tranilast on the release of arachidonic acid and its metabolites. Jpn J Pharmacol. 1988;46: 53-60. 47. Tamai H, Katou K, Hayakawa H, et al. The impact of tranilast on restenosis following coronary angioplasty: the second tranilast restenosis following angioplasty trial (TREAT-2) (Abstract). Circulation. 1996;94(suppl I):I-620. Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933430/ on 05/10/2017