* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Eye induction
Survey
Document related concepts
Transcript
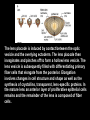
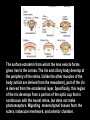
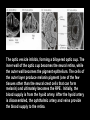
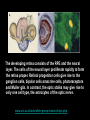
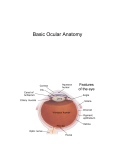
Eye induction and retinal cell specification Chris Strang Ph.D. Vision Sciences WORB 308 [email protected] 975-7222 Outline • Eye field and optic vesicle induction • Development of the lens, iris, ciliary body, and anterior chamber. • Retina induction • Retinal cell proliferation – Birth order of retinal cell types – Developmental potency – Intrinsic and extrinsic factors influencing cell fate • Specification of fovea Eye field induction: The eye field begins to be defined as early as the morula (32 cell) stage. In morula stage frog embryos, only a subset of cells is competent to become retina. Wnt signaling is required for diencephalic fate. It is also specifically required for eye induction. Wnt signaling causes expression of the eyespecific transcription factors Pax6 and Rx1. These, along with Six3, are initially expressed in a single domain across the anterior neural plate of many species. Overexpression of the Wnt receptor ‘frizzled’ results in overexpression of Pax6 and Rx1. The separation of the single domain into two bilateral eye fields depends upon Shh. Shh protein from the prechordal plate suppresses Pax6 expression in the center of the embryo, dividing the field in two. Mutations in the SHH gene or inhibition of protein processing results in cyclopia. The phenotype results in a single eye in the center of the face (and usually below the nose). The primary optic vesicles arise from the frontal eye fields as an evagination of neural tube epithelium at the 5 vesicle stage. The optic vesicle is connected to the diencephalon by the optic stalk, which will become the optic nerve. The eye arises from several types tissues. Neural ectoderm gives rise to retina and retinal pigment epithelium (RPE), the lens is derived from surface ectoderm, while the sclera and anterior chamber are derived from migrating cells. In humans, eye development begins around E22, but isn’t complete until several months after birth. The lens placode is induced by contact between the optic vesicle and the overlying ectoderm. The lens placode then invaginates and pinches off to form a hollow lens vesicle. The lens vesicle is subsequently filled with differentiating primary fiber cells that elongate from the posterior. Elongation involves changes in cell structure and shape as well as the synthesis of crystallins, transparent, lens-specific proteins. In the mature lens an anterior layer of proliferative epithelial cells remains and the remainder of the lens is composed of fiber cells. The surface ectoderm from which the lens vesicle forms gives rise to the cornea. The iris and ciliary body develop at the periphery of the retina. Unlike the other muscles of the body (which are derived from the mesoderm), part of the iris is derived from the ectodermal layer. Specifically, this region of the iris develops from a portion of the optic cup that is continuous with the neural retina, but does not make photoreceptors. Migrating mesenchymal tissues form the sclera, trabecular meshwork, and anterior chamber. The optic vesicle infolds, forming a bilayered optic cup. The inner wall of the optic cup becomes the neural retina, while the outer wall becomes the pigment epithelium. The cells of the outer layer produce melanin pigment (one of the few tissues other than the neural crest cells that can form melanin) and ultimately becomes the RPE. Initially, the blood supply is from the hyoid artery. After the hyoid artery is disassembled, the ophthalmic artery and veins provide the blood supply to the retina. The role of Pax6 in eye formation is conserved across many species, including human and fruit fly. If the mouse Pax6 gene is inserted into the fruit fly genome and activated randomly, eyes form in cells where mouse Pax6 is expressed. The Pax6 protein remains important in the development of the lens and retina. It is also expressed in the mouse forebrain, hindbrain, and nasal placodes. However, the eyes seem to be most sensitive to its absence. Pax6 knockouts lack eyes, and heterozygotes have small eyes. The developing retina consists of the RPE and the neural layer. The cells of the neural layer proliferate rapidly to form the retina proper. Retinal progenitor cells give rise to the ganglion cells, bipolar cells amacrine cells, photoreceptors and Muller glia. In contrast, the optic stalks may give rise to only one cell type, the astrocytes of the optic nerve. www.ucl.ac.uk/zebrafish-group/research/eye.php One of the first steps in retina formation is to enlargement of the progenitor pool. S-phase: DNA synthesis/chromosome duplication G2: interphase 2 M-phase: mitotis G1: interphase 1 G0: terminal division The outer surface, the ventricular zone, is apposed to the RPE, while the inner layer is at the vitreal side. As with developing cortex, prolifererating cell undergo interkinetic nuclear migration. Cell division takes place at the outer ventricular surface. During G1 cells migrate inward. They undergo S-phase at the inner, vitreal surface, and migrate outward in G2. Cells that exit the cell cycle do so at the outer surface, and then migrate to final location. Although cells leave the cell cycle at the outer surface, retinal layers are generated from the inner retina to the outer. The GCL arises first, followed by the INL and then the ONL. Cells destined for each of these layers may be born concurrently, but timing influences the probability of a cell taking on one fate or another. Fate may be defined by cell ‘birthday’. Cells may be fate committed at the point they leave the cell cycle. Competence becomes more restricted as development progresses. Ganglion cells are born first. Small GCs are born earliest, and large GCs are later. GCs whose axons will not cross the optic chiasm are born earlier than those that will develop axons that will cross contralaterally. GCs are followed by the development of horizontal cells and then cones. Cone genesis usually begins and ends before rod genesis. Amacrine cells, bipolar cells, rods and Müller glia cells are born later. Different AC types may also be born at different times. For example, amacrine cells born earliest may migrate to the GCL, and give rise to the population of displaced amacrine cells. The birth orders of cones and rods varies slightly by species and whether or not the retina is rod or cone dominated. If more of a given cell type is needed, that type may be born later. This allows more progenitors to be formed prior to exiting the cell cycle. Retinal progenitor cells are multipotent. A single progenitor cell can give rise to each of the cell types in the retina. However, competence may become increasingly restricted over time. That is, specific fates may only be available at different time periods. There appears to be a combination of internal programming at time of birth and environmental exposure. There is a balance between intrinsic and extrinsic factors that influence cell fate. Intrinsic factors: The primary intrinsic factor is birthday. Cells dissociated at the time that GCs are usually born tend to become RGCs. That is, progenitor cells dissociated early in development have a higher probability of differentiating into GCs. In contrast, cells that are dissociated later in development have a higher probability of differentiating into rods. While there are interactions between multiple intrinsic factors, the importance of a cell’s birthday appears to be due to transcription factors expressed at different time points. Expression of members of bHLH proneural genes appear to drive specific cell fate. For example, Chx10 promotes bipolar cells, while Prox1 is involved in HC fate Extrinsic factors: Feedback from postmitotic neurons is a primary example. If early embryonic retinal progenitors are cultured with other early progenitors, they tend to differentiate into GCs. However, if early cells are either mixed with late progenitors, or exposed to media from cultures of later progenitors they tend to take on the later fate. Notch-delta signaling is another extrinsic feedback mechanism. Inactive notch promotes neuronal differentiation and upregulates the ligand delta. Delta from a differentiated neural cell binds and activates notch in a neighboring cell. The active notch delays differentiation of the second cell which increases the likelihood of it becoming a Müller cell. Development of the fovea: The primate fovea is specialized for high acuity color vision. It is avascular, with an extremely high density of cones and an absence of inner retinal layers, due to displacement to the periphery. Rods are also absent from the center 300 mm and S-cones are sparse in the central 100 mm . Although the fovea is thinner overall, the ONL over the center of the foveal pit is very thick, due to the high density of cone cell bodies. The cones are very long and thin. The axons, fibers of Henle, extend away from fovea. There is also a bias towards cells of the midget pathway which is responsible for detailed color vision. During development, all of the cell types are produced, except for rods and possibly S-cones. Over time the cones in the fovea centralis become more tightly packed, while the GCL, IPL and INL are moved toward periphery. This causes a thickening of the inner layers around the pit and forms the foveal rim. The long fibers of Henle serve to maintain the connections between photoreceptors, horizontal cells and bipolar cells. Retinal cell differentiation begins in the center and progresses to periphery. The order of cell differentiation is same and synaptic connections are formed. Thus, prior to pit formation, the central retina has all five layers. At the beginning of fovea differentiation, packing density in the cone foveal mosaic is 12,000/mm2, by the time the fovea has matured packing density is 30,000/mm2 . This occurs via displacement not generation of new cells. Pre- pit stage: The OPL is thin, while the INL IPL, and GCL are thick. At this point the early fovea is thicker than the rest of the retina, because the peripheral retina is not as far advanced developmentally. There is a central bulge and rods can be identified surrounding the cone mosaic. Structurally, the cone pedicle is adjacent to the cell nucleus, and only a short axon is needed to make synaptic connections in the OPL. The foveal depression begins to form as the GCL and IPL are displaced toward the periphery and axons from the INL to the GCL are angled to maintain synaptic connections. The INL still has several layers of cell bodies. The ONL remains thin with only short Henle fibers from the ONL to the OPL. As the INL thins and cells are displaced to the sides, the photoreceptor cells become packed more tightly in the fovea. At birth, the human fovea is still incompletely developed. Cone packing results important morphological changes. Cone cell bodies and the fibers of Henle elongate. Initially cones are cuboidal and 8-10 mm in diameter. At birth (for people), the synaptic pedicle and the inner and outer segments have developed, and the cell has become ~11-14 mm in length. Adult foveal cones will be 80-100 mm long and the diameter will decrease to ~2 mm. Cones tilt so the that long end is tipped toward the center, and the Henle fiber is angled away from the center to maintain OPL connections. Outline • Eye field and optic vesicle induction • Development of the lens, iris, ciliary body, and anterior chamber. • Retina induction • Retinal cell proliferation – Birth order of retinal cell types – Developmental potency – Intrinsic and extrinsic factors influencing cell fate • Specification of fovea