* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download NWQA240_3_miconazole_gel_statins_interaction
Survey
Document related concepts
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Drug design wikipedia , lookup
Drug discovery wikipedia , lookup
National Institute for Health and Care Excellence wikipedia , lookup
Pharmacognosy wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Sol–gel process wikipedia , lookup
Prescription costs wikipedia , lookup
Dydrogesterone wikipedia , lookup
Transcript
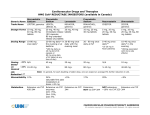
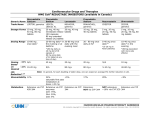
Medicines Q&As Q&A 240.3 Can miconazole oral gel be used by patients taking a statin? Prepared by UK Medicines Information (UKMi) pharmacists for NHS healthcare professionals Before using this Q&A, read the disclaimer at www.ukmi.nhs.uk/activities/medicinesQAs/default.asp Date prepared: April 2014 Summary Miconazole is an azole antifungal which inhibits cytochrome P450 isoenzymes CYP2C9 and CYP3A4. It is absorbed systemically from the oral gel preparation and has the potential to raise plasma levels of drugs metabolised by these isoenzymes, increasing the risk of adverse effects. The Summary of Product Characteristics for miconazole oral gel (Daktarin) contraindicates the coadministration of miconazole with drugs that are metabolised by CYP3A4, including some of the statins. There are no reports describing interactions between miconazole and any of the statins. Patients taking pravastatin can use miconazole oral gel. Pravastatin is not metabolised by CYP450 isoenzymes and therefore will not interact with miconazole. As metabolism via CYP3A4 is limited for fluvastatin and rosuvastatin, a clinically significant interaction is unlikely. Miconazole oral gel could be used with caution in patients taking fluvastatin or rosuvastatin, provided they are monitored for adverse effects. For patients taking atorvastatin or simvastatin, the lack of reports of an interaction with miconazole oral gel suggests that the antifungal may be used with caution if considered essential and there is no suitable alternative. However, cases of rhabdomyolysis have been reported with concomitant use of fluconazole and atorvastatin or simvastatin, and as miconazole has the potential to interact similarly, prescribers should consider the benefits of treatment versus the risk of using the combination. Prescribers should be aware that prescribing the combination of atorvastatin, simvastatin, fluvastatin or rosuvastatin and Daktarin is outside of the product licence. Any patient taking a statin (other than pravastatin) who is given miconazole oral gel should be warned to watch for signs of myopathy (i.e. unexplained muscle pain, tenderness or weakness or dark coloured urine). If myopathy does occur the statin should be stopped immediately. Background Miconazole is an azole antifungal used for the treatment and prevention of oral and gastrointestinal fungal infections [1]. It is available as an oral gel which can be applied topically to lesions or ingested at a dose of 5 to 10ml four times daily for gastrointestinal infections [1, 2]. The Summary of Product Characteristics (SmPC) for miconazole oral gel (Daktarin) contraindicates coadministration of miconazole with drugs that are metabolised by the cytochrome P450 isoenzyme CYP3A4 including HMG-CoA reductase inhibitors (statins), such as simvastatin [1]. This Medicines Q&A explores the evidence for the interaction between miconazole oral gel and the statins. Answer When miconazole oral gel is applied locally, a large proportion may be swallowed or absorbed through inflamed oral mucosa and therefore absorbed systemically [3]. Miconazole oral gel has been reported to increase the INR in patients taking warfarin [4,5], indicating that it is absorbed systemically in sufficient amounts to result in clinically significant drug interactions. The ability of azole antifungals to inhibit cytochrome P450 isoenzymes is variable. Miconazole and fluconazole inhibit CYP3A4 and CYP2C9. Itraconazole and ketoconazole are potent inhibitors of CYP3A4 (but not CYP2C9). Of the statins, atorvastatin and simvastatin are metabolised by CYP3A4 [6,7]. Fluvastatin is substantially metabolised by CYP2C9 and a small amount is metabolised by CYP3A4 [2], whilst Available through NICE Evidence Search at www.evidence.nhs.uk rosuvastatin undergoes limited metabolism by CYP2C9 and by CYP3A4 to a lesser extent [8]. Pravastatin is not metabolised to a clinically significant extent by the CYP450 system [9]. The Medicines and Healthcare products Regulatory Agency (MHRA) acknowledges the potential for interaction between some of the statins and azole antifungals. It issued guidance in January 2008 advising that prescribing of potent inhibitors of CYP3A4, such as itraconazole and ketoconazole, should be avoided with atorvastatin and simvastatin [10]. However they made no recommendation to avoid other azole antifungals such as miconazole. In addition, administration of miconazole oral gel is not listed as a contraindication in the SmPCs for any of the statins [6-9,11]. What data are available to support an interaction between azoles and statins? Miconazole There do not appear to be any reports of an interaction between miconazole and any of the statins. However, like fluconazole, miconazole is an inhibitor of CYP3A4 and CYP2C9, and might be expected to interact in a similar way [2,4]. Fluconazole Rhabdomyolysis in patients taking a statin and fluconazole has been reported [4]. A patient taking simvastatin 40mg daily developed muscle weakness and elevated serum creatinine kinase one week after starting fluconazole 400mg daily. Two similar cases of rhabdomyolysis have been reported in patients taking simvastatin and fluconazole. In both cases rhabdomyolysis resolved after discontinuing fluconazole [4]. Myopathy and rhabdomyolysis resulting in multiple organ failure and death occurred in a patient taking atorvastatin and fluconazole. The patient was previously taking pravastatin 80mg daily and fluconazole 150mg daily. Within one week of switching to atorvastatin 40mg he developed myopathy, rhabdomyolysis and renal failure and later died; an interaction between the two drugs was considered the cause [4]. Administration of fluconazole (400mg on day 1 followed by 200mg for three days) to 12 healthy subjects taking fluvastatin 40mg resulted in an 84% increase in the AUC of fluvastatin and increased plasma levels by 44% [4]. Fluconazole administration to 14 subjects resulted in a very small rise in the AUC and plasma concentrations of rosuvastatin. No clinically significant adverse effects were reported [4]. Itraconazole Studies have shown the potential for itraconazole to increase the AUC of simvastatin from 1.5 to more than 20 fold. High interpatient variability puts certain patients at much greater risk than others [12]. Two cases of rhabdomyolysis have been reported in patients taking simvastatin. The first was a 74 year old man who was taking simvastatin 40mg daily. Within three weeks of starting itraconazole 200mg daily, his muscles became tender and serum creatinine kinase levels were elevated. A second patient was also taking simvastatin 40mg daily and developed symptoms of rhabdomyolysis within two weeks of starting itraconazole 100mg twice daily. Several futher cases of raised simvastatin serum levels have been reported in patients who have started taking itraconazole [4]. Itraconazole has been shown to result in marked rises in atorvastatin plasma levels, but no change in fluvastatin [4]. Concomitant administration of itraconazole and rosuvastatin resulted in a small but clinically insignificant rise in rosuvastatin plasma levels [8]. Ketoconazole As ketoconazole is also a potent inhibitor of CYP3A4, it is expected to interact in the same way as itraconazole. Five cases of rhabdomyolysis have been reported in patients taking simvastatin, which developed between seven days and four weeks after starting ketoconazole [4]. What are the implications of using miconazole oral gel in patients taking statins? There are no data available that confirm that concomitant use of miconazole and any of the statins results in a clinically significant interaction. The basis for the manufacturer’s contraindication appears to be extrapolation from reports of interactions between other azoles and statins. Interactions are theoretically possible with all statins except pravastatin, but are unlikely, especially with fluvastatin and rosuvastatin for which metabolism via CYP3A4 is less important. Available through NICE Evidence Search at www.evidence.nhs.uk Pravastatin Patients taking pravastatin can use miconazole oral gel as no interaction is expected [4, 9]. Fluvastatin and rosuvastatin Fluvastatin is predominantly metabolised by CYP2C9 and less so by CYP3A4 so theoretically is less likely to interact with miconazole [11,12]. However, miconazole is an inhibitor of CYP2C9 so a degree of caution is warranted [4]. Rosuvastatin undergoes limited metabolism. Approximately 10% is metabolised, principally by CYP2C9. The manufacturer of rosuvastatin states that drug interactions resulting from CYP450-mediated metabolism are not expected [8]. Patients taking fluvastatin or rosuvastatin can use miconazole oral gel. However, they should be advised to report any signs of myopathy and possible rhabdomyolysis (i.e. unexplained muscle pain, tenderness or weakness or dark coloured urine). If myopathy does occur, the statin should be stopped immediately [4]. Atorvastatin and simvastatin Both atorvastatin and simvastatin are metabolised by CYP3A4 [13] and the SmPC for miconazole oral gel (Daktarin) contraindicates use of the gel in patients taking these statins. Lack of reports of any interaction with miconazole suggests that the combination would be safe to use if considered essential and there is no other suitable alternative. However, cases of rhabdomyolysis have been reported with fluconazole, and as miconazole has the potential to interact similarly [4], prescribers should consider the benefits of treatment versus the risk of using the combination. Patients taking atorvastatin or simvastatin should use miconazole oral gel with caution [12]. They should be monitored for adverse effects and advised to report any signs of myopathy and possible rhabdomyolysis (i.e. unexplained muscle pain, tenderness or weakness or dark coloured urine). If myopathy does occur, the statin should be stopped immediately. For those taking high doses of atorvastatin (i.e. 80mg), consider temporarily reducing the dose or even stopping treatment during the course of miconazole [6, 10]. If long term antifungal treatment is needed, it would seem safer to prescribe a non-azole antifungal agent for patients taking atorvastatin or simvastatin. Prescribing issues As the SmPC for miconazole oral gel (Daktarin) contraindicates the coadministration of drugs metabolised by CYP3A4, this would exclude patients taking atorvastatin, simvastatin, fluvastatin and rosuvastatin from using the gel. However, if no suitable alternative exists it may be necessary to prescribe miconazole in combination with a statin. Prescribers should be aware that by doing this they will be prescribing Daktarin outside of the product licence and will take responsibility for their decision to do this should any adverse effects occur. If adverse effects do occur, these should be reported to the MHRA via the Yellow Card Scheme. Limitations Data for drug interations with miconazole are limited. Most data available are for azoles other than miconazole. References 1. Summary of Product Characteristics – Daktarin oral gel. Janssen-Cilag Ltd. Date of last revision of text 5/12/13. Accessed via www.medicines.org.uk on 01/04/14. 2. Sweetman, S (Ed), Martindale: The Complete Drug Reference. London. Pharmaceutical Press. Accessed via www.medicinescomplete.com on 01/04/14. 3. Khanderia S, editor. Joint Formulary Committee. British National Formulary. 67th ed. London: British Medical Association and Royal Pharmaceutical Society of Great Britain. March 2014. 4. Baxter K, editor. Stockley’s Drug Interactions. London: Pharmaceutical Press. Accessed via www.medicinescomplete.com on 01/04/14. 5. Australian Adverse Drug Reactions Bulletin. Miconazole oral gel elevates INR; December 2002, Volume 21, number 4. 6. Summary of Product Characteristics – Lipitor. Pfizer Ltd. Date of last revision of text 08/13. Accessed via www.medicines.org.uk on 01/04/14. Available through NICE Evidence Search at www.evidence.nhs.uk 7. Summary of Product Characteristics – Zocor. Merck Sharp & Dohme Ltd. Date of last revision of text 11/13. Accessed via www.medicines.org.uk on 01/04/14. 8. Summary of Product Characteristics – Crestor. Astra Zeneca UK Ltd. Date of last revision of text 23/12/13. Accessed via www.medicines.org.uk on 01/04/14. 9. Summary of Product Characteristics – Lipostat. Bristol-Myers Squibb Pharmaceuticals Ltd. Date of last revision of text 24/04/13. Accessed via www.medicines.org.uk on 01/04/14. 10. Medicines and Healthcare products Regulatory Agency – Drug Safety Update; January 2008, Volume 1, issue 6. 11. Summary of Product Characteristics – Lescol. Novartis Pharmaceutical UK Limited. Date of last revision of text 01/11/13. Accessed via www.medicines.org.uk on 01/04/14. 12. White CM. An evaluation of CYP3A4 drug interactions with HMG-CoA reductase inhibitors. Formulary 2000; 35: 343-52. 13. Gubbins PO. Triazole antifungal agents drug-drug interactions involving hepatic cytochrome P450. Expert Opin Drug Metab Toxicol 2011; 7: 1411-29. Bibliography The following resources were used for background reading: Cytochrome P450 Drug Interaction Tables. Indiana University: School of Medicine, Division of Pharmacology. Accessed via http://www.medicine.iupui.edu/clinpharm on 01/04/14. Martin J, Fay M. Cytochrome P450 drug interactions: are they clinically relevant? Aust Prescr 2001; 24: 10-2. Zhang W, Ramamoorthy Y, Kilicarslan T et al. Inhibition of cytochromes P450 by antifungal imidazole derivatives. Drug Metab Dispos 2002: 30; 314-18. Sweetman S (Ed) Martindale: The Complete Drug Reference, London: Pharmaceutical Press. Itraconazole, ketoconazole, miconazole, fluconazole monographs. Accessed via www.medicinescomplete.com on 01/04/14. Scheen AJ. Cytochrome P450-mediated cardiovascular drug interactions. Expert Opin Drug Metab Toxicol 2011; 7: 1065-82. Quality Assurance Prepared by Simone Henderson. North West Medicines Information Centre, Liverpool. Date Prepared March 2010 This version prepared April 2014 Checked by Joanne McEntee. North West Medicines Information Centre, Liverpool. Christine Proudlove. North West Medicines Information Centre, Liverpool. Date of check March 2010 This version checked April 2014 Search strategy Embase: Miconazole + [HMG-COA reductase inhibitors or atorvastatin or fluvastatin or pravastatin or rosuvastatin or simvastatin]. Embase: CYTOCHROME P450 + [FLUCONAZOLE OR ITRACONAZOLE OR PYRROLE DERIVATIVE OR KETOCONAZOLE OR CLOTRIMAZOLE OR MICONAZOLE OR VORICONAZOLE]. Searched limited to Review and English language. Medline: Miconazole + [HMG-COA reductase inhibitors or atorvastatin or fluvastatin or pravastatin or rosuvastatin or simvastatin]. Available through NICE Evidence Search at www.evidence.nhs.uk Medline: AZOLES + CYTOCHROME P-450 ENZYME SYSTEM. Review and English language Electronic Medicines Compendium – Summary of Product Characteristics for Daktarin oral gel, simvastatin, atorvastatin, rosuvastatin, pravastatin, fluvastatin. Reactions Weekly www.adisonline.com searched: Miconazole + [atorvastatin or fluvastatin or pravastatin or rosuvastatin or simvastatin]. In-house database/ resources Available through NICE Evidence Search at www.evidence.nhs.uk