* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Suggestion from clinicians
Adherence (medicine) wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug interaction wikipedia , lookup
Clinical trial wikipedia , lookup
Neuropharmacology wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Psychopharmacology wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacogenomics wikipedia , lookup
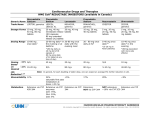
Simvastatin vs. Rosuvastatin vs. Atorvastatin Replacement to the List Peer Feedback: “Most folks now favour using the high potency statins. Atorvastatin or rosuvastatin. Suggest replacing simvastatin with these.” “same interactions as atorva, rosuva more potent LDL lowering, simva 80 mg is not recommended" Rosuvastatin was also suggested as an addition: “One of the more commonly used statins” Note: Atorvastatin is on the list. Literature Review Question: Which of Rosuvastatin, atorvastatin and simvastatin is the most efficacious? Literature Search: Cochrane Review Search of each drug Pubmed: ‘rosuvastatin AND atorvastatin AND simvastatin AND efficacy AND comparison’ Pubmed: ‘rosuvastatin AND simvastatin AND efficacy AND comparison’ eCPS - Cardiovascular Disorders: Dyslipidemias Atorvastatin Cochrane Review (2015) In this update, we found an additional 42 trials and added them to the original 254 studies. The update consists of 296 trials that evaluated dose-related efficacy of atorvastatin in 38,817 participants. Included are 242 before-and-after trials and 54 placebo-controlled RCTs. Log dose-response data from both trial designs revealed linear dose-related effects on blood total cholesterol, LDL-cholesterol, HDL-cholesterol and triglycerides. The Summary of findings table 1 documents the effect of atorvastatin on LDLcholesterol over the dose range of 10 to 80 mg/d, which is the range for which this systematic review acquired the greatest quantity of data. Over this range, blood LDL-cholesterol is decreased by 37.1% to 51.7% (Summary of findings table 1). The slope of dose-related effects on cholesterol and LDLcholesterol was similar for atorvastatin and rosuvastatin, but rosuvastatin is about three-fold more potent. Subgroup analyses suggested that the atorvastatin effect was greater in females than in males and was greater in non-familial than in familial hypercholesterolaemia. Risk of bias for the outcome of withdrawals due to adverse effects (WDAEs) was high, but the mostly unclear risk of bias was judged unlikely to affect lipid measurements. Withdrawals due to adverse effects were not statistically significantly different between atorvastatin and placebo groups in these short-term trials (risk ratio 0.98, 95% confidence interval 0.68 to 1.40). This update resulted in no change to the main conclusions of the review but significantly increases the strength of the evidence. Studies show that atorvastatin decreases blood total cholesterol and LDLcholesterol in a linear dose-related manner over the commonly prescribed dose range.New findings include that atorvastatin is more than three-fold less potent than rosuvastatin, and that the cholesterollowering effects of atorvastatin are greater in females than in males and greater in non-familial than in familial hypercholesterolaemia. This review update does not provide a good estimate of the incidence of harms associated with atorvastatin because included trials were of short duration and adverse effects were not reported in 37% of placebo-controlled trials. Adams, Stephen P., Michael Tsang, and James M. Wright. "Lipid lowering efficacy of atorvastatin." The Cochrane Library (2012). Rosuvastatin Cochrane review 2014 One-hundred and eight trials (18 placebo-controlled and 90 before-and-after) evaluated the dose-related efficacy of rosuvastatin in 19,596 participants. Rosuvastatin 10 to 40 mg/day caused LDL-cholesterol decreases of 46%to 55%, when all the trials were combined using the generic inverse variance method. The quality of evidence for these effects is high. Log dose-response data over doses of 1to 80 mg, revealed strong linear dose-related effects on blood total cholesterol, LDL-cholesterol and non-HDLcholesterol. When compared to atorvastatin, rosuvastatin was about three-fold more potent at reducing LDL-cholesterol. There was no dose-related effect of rosuvastatin on blood HDL-cholesterol, but overall, rosuvastatin increased HDL by 7%. There is a high risk of bias for the trials in this review, which would affect WDAEs, but unlikely to affect the lipid measurements. WDAEs were not statistically different between rosuvastatin and placebo in 10 of 18 of these short-term trials (risk ratio 0.84; 95% confidence interval 0.48 to 1.47). The total blood total cholesterol, LDL-cholesterol and non-HDL-cholesterol-lowering effect of rosuvastatin was linearly dependent on dose. Rosuvastatin log dose-response data were linear over the commonly prescribed dose range. Based on an informal comparison with atorvastatin, this represents a three-fold greater potency. This review did not provide a good estimate of the incidence of harms associated with rosuvastatin because of the short duration of the trials and the lack of reporting of adverse effects in 44%of the placebo-controlled trials. Adams, Stephen P., Sarpreet S. Sekhon, and James M. Wright. "Lipid‐lowering efficacy of rosuvastatin." The Cochrane Library (2014). Rosuvastatin vs Atorvastatin, Pravastatin, and Simvastatin. 2014 More recently, various studies7,18 reported that patients with MetS had greater reductions in TG and somewhat greater percentage increases in HDL-C with statin treatment, as expected. The comparisons between statin treatment groups showed consistent advantages of rosuvastatin treatment, compared with atorvastatin, simvastatin, and pravastatin, in LDL-C goal achievement and in LDL-C, total cholesterol, and non-HDL-C reduction. As in the main study analysis, rosuvastatin 10 mg provided benefits comparable to a higher dose of atorvastatin in the MetS population. It is worth noting that a pharmacoeconomic analysis of the primary MERCURY results7,18 showed that treatment with rosuvastatin 10 mg was more costeffective compared with equivalent or higher doses of atorvastatin, simvastatin, and pravastatin, and that switching patients from a comparator statin to rosuvastatin improved LDL-C goal attainment at relatively little additional cost, with equivalent (or lower) associated drug costs.7,18 Thus, rosuvastatin may have pharmacoeconomic advantages, compared with atorvastatin, while providing comparable efficacy. Finally, rosuvastatin at a low dose has demonstrated high efficacy for LDL-C lowering, enabling patients with hypercholesterolemia to achieve their lipid goals.13,18 In addition, rosuvastatin has beneficial effects on other components of the lipid profile, including HDL-C,13,24-27 which is a major, independent risk factor for CVD.8,25 Safety data from several large-scale clinical and pharmacoepidemiologic studies have shown that the safety of rosuvastatin25-27 and results from the current recent study also support these findings.19 Bener, Abdulbari, et al. "Comparison of Cost-Effectiveness, Safety, and Efficacy of Rosuvastatin Versus Atorvastatin, Pravastatin, and Simvastatin in Dyslipidemic Diabetic Patients With or Without Metabolic Syndrome." Journal of primary care & community health 5.3 (2014): 180-187. Mckenney et al 2003 The STELLAR trial is the largest trial of its kind to date to compare dose-related effects of statins on lipid goal achievement in patients with hypercholesterolemia. Trial results indicate that rosuvastatin 10 to 40 mg has greater efficacy than atorvastatin 10 to 80 mg, simvastatin 10 to 80 mg, and pravastatin 10 to 40 mg for achievement of ATP III LDL-C and non-HDL-C goals, European LDL-C goals, and Canadian LDLC and triple goals (which also included total cholesterol/HDL-C ratio and triglyceride goals). It is particularly noteworthy that with rosuvastatin therapy, more patients achieve the most aggressive LDL-C goals of < 100 mg/dl (< 2.6 mmol/l) and < 116 mg/dl (< 3.0 mmol/l), regardless of CHD risk status. This greater goal-attaining efficacy of rosuvastatin is due in large part to its greater efficacy in lowering LDL-C, as shown previously (Figure 7)14. Rosuvastatin 10 mg reduced LDL-C by 46%, which was significantly greater ( p < 0.002) than the 37% reduction achieved with atorvastatin 10 mg, the 28% to 39% reductions achieved with simvastatin 10 to 40 mg, and the 20% to 30% reductions achieved with pravastatin 10 to 40 mg14. In the rosuvastatin 40-mg group, LDL-C was reduced by 55%, compared with 48% for atorvastatin 40 mg ( p < 0.002), 51% for atorvastatin 80 mg ( p = 0.006, NS), 39% for simvastatin 40 mg ( p < 0.002), 46% for simvastatin 80 mg ( p < 0.002), and 30% for pravastatin 40 mg ( p < 0.002)14. In summary, more patients treated with rosuvastatin 10 to 40mg achieved lipid goals, and optimal LDL-C levels as suggested by the NCEP ATP III and European guidelines, than patients treated with atorvastatin, simvastatin, and pravastatin. The proportions of patients in the rosuvastatin 10-mg group who reached the LDL-C level of < 100 mg/dl (< 2.6 mmol/l), both the non-HDL-C and LDL-C goals, and the Canadian LDL-C goals were significantly greater ( p < 0.002) than the proportions in the atorvastatin 10-mg, simvastatin 10-, 20-, and 40-mg, and pravastatin 10-, 20-, and 40-mg groups. The highest proportions of patients who achieved NCEP ATP III, European, and Canadian goals were in the rosuvastatin groups. McKenney, James M., et al. "Comparison of the efficacy of rosuvastatin versus atorvastatin, simvastatin, and pravastatin in achieving lipid goals: results from the STELLAR trial." Current Medical Research and Opinion® 19.8 (2003): 689-698. eCPS (2014) Class Drug HMG-CoA Reductase Inhibitors atorvastatin Lipitor, generics HMG-CoA Reductase Inhibitors rosuvastatin Crestor, generics Administer antacids 2 h after rosuvastatin. HMG-CoA Reductase Inhibitors simvastatin Zocor, generics Adverse Effects Drug Interactions Adults: 10–80 mg daily po at any time of day Children:b 10– 20 mg daily po Common: Increased CK, Increased transaminases (reversible), mild upper GI disturbances, myalgias (with and without CK elevation), sleep disturbances, headache, rash. Rare: myopathy, rhabdomyolysis, peripheral neuropathy, lupus-like symptoms, impotence. Avoid with CYP3A4 inhibitors: amiodarone, azoles, cyclosporine, gemfibrozil, grapefruit juice, macrolide antibiotics, nondihydropyridine calcium channel blockers, e.g., verapamil, protease inhibitors. Common: Increased CK, Increased transaminases (reversible), mild upper GI disturbances, myalgias (with and without CK elevation), sleep disturbances, headache, rash. Rare: myopathy, rhabdomyolysis, peripheral neuropathy, lupus-like symptoms, impotence. Avoid with CYP2C9 inhibitors: amiodarone, fluconazole, fluoxetine, fluvoxamine. Reduced levels with Contraindications: active concomitant use of liver disease, high alcohol magnesium/aluminum consumption, pregnancy. hydroxide-containing antacids. $ Common: Increased CK, Increased transaminases (reversible), mild upper GI disturbances, myalgias (with and without CK elevation), sleep disturbances, headache, rash. Rare: myopathy, rhabdomyolysis, peripheral neuropathy, lupus-like symptoms, impotence. Avoid with CYP3A4 inhibitors: amiodarone, azoles, cyclosporine, gemfibrozil, grapefruit juice, macrolide antibiotics, nondihydropyridine calcium channel blockers, e.g., verapamil, protease inhibitors. $ Adults: 10–40 mg daily po Initial dose 10 mg/day po except in Asian patients and those receiving cyclosporine (initial dose 5 mg/day po) Comments Costa Dose Start with low doses and $ titrate up to reach targets while monitoring biochemical markers. Liver function (ALT) should be checked at least once at 3 months. Check CK if myalgia develops. Use caution in patients with moderate to severe renal impairment(<60 mL/min). Children:b 5–10 mg daily po Adults: 10–80 mg po with evening meal Children:b 10– 40 mg po with evening meal Cardiovascular Disorders: Dyslipidemias; Ghislaine O. Roederer, MD, PhD; Date of revision: June 2014 Medication simvastatin atorvastatin Uses Contraindications (CI), drug interactions (DI) or cautions Hyperlipidemia, coronary artery disease, heart failure CI: active liver disease, alcohol and pregnancy hyperlipidemia, coronary artery disease, heart failure CI: active liver disease, alcohol, pregnancy DI: CPY-3A4, digoxin, warfarin, clarithromycin, erythromycin, gemfibrozil, grapefruit juice, verapamil DI: digoxin, warfarin, clarithromycin, erythromycin, gemfibrozil, grapefruit juice, verapamil Adverse Effects (common and severe) increased liver function tests, myopathy, rhabdomyolysis increased liver function tests, myopathy, rhabdomyolysis Initial dose; typical dose Monitoring 20mg; 2080mg one time a day / at nights international normalized ratio (INR) 10, 40mg; 1040mg one time a day LFTs, creatinine kinase (CK)