* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Transcript
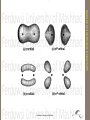
Advanced Inorganic Chemistry ADVANCED INORGANIC CHEMISTRY UV visible infrared 3A 3A 2g →1Eg [Ni(NH3)6]2+ υ, cm-1 2g →3T2g ? ~10-18 J • Electronic transitions • UV and visible wavelengths • Molecular vibrations • Thermal infrared wavelengths Increasing energy • Molecular rotations • Microwave and far-IR wavelengths ~10-23 J • Each of these processes is quantized • Translational kinetic energy of molecules is unquantized ADVANCED INORGANIC CHEMISTRY MOLECULAR ABSORPTION PROCESSES Electronic XPS UPS UV-visible Ferdowsi University of Mashhad 5 ADVANCED INORGANIC CHEMISTRY ترم طیفی ELECTRONIC (UV-VISIBLE) SPECTROSCOPY c=n.l With energy of photons E=h.n Ferdowsi University of Mashhad 6 ADVANCED INORGANIC CHEMISTRY ترم طیفی ELECTRONIC (UV-VISIBLE) SPECTROSCOPY (2) charge transfer metal-ligand (MLCT) ligand-metal (LMCT) ADVANCED INORGANIC CHEMISTRY (1) metal-metal (d-d) transition ترم طیفی UV-visible spectroscopy ligand p* metal d s* metal d n ligand p n (3) ligand-centered transition s s s*, n s*, n p*, and p p* Ferdowsi University of Mashhad 7 ADVANCED INORGANIC CHEMISTRY ترم طیفی UV-visible spectroscopy (1) metal-metal (d-d) transition (2) charge transfer metal-ligand (MLCT) ligand-metal (LMCT) (3) ligand-centered transition s s*, n s*, n p*, and p p* Ferdowsi University of Mashhad 8 ADVANCED INORGANIC CHEMISTRY Ferdowsi University of Mashhad 9 single bonds → sigma (s) orbitals → s electrons double bond → a sigma (s) orbital and a pi (p) molecular orbital Pi orbitals are formed by the parallel overlap of atomic p orbitals Ferdowsi University of Mashhad 10 ADVANCED INORGANIC CHEMISTRY There are three types of electronic transitions: - p, s, and n electrons - d and f electrons - charge transfer electrons Selection Rules 1. Spin selection rule: DS = 0 only one electron is involved in any transition allowed transitions: singlet singlet or triplet triplet forbidden transitions: singlet triplet or triplet singlet Changes in spin multiplicity are forbidden • Spin-forbidden transitions – Transitions involving a change in the spin state of the molecule are forbidden – Strongly obeyed – Relaxed by effects that make spin a poor quantum number (heavy atoms) Selection rules 2. Laporte selection rule (or parity rule): there must be a change in the parity (symmetry) of the complex DL = ±1 Electric dipole transition can occur only between states of opposite parity. Laporte-allowed transitions: gu or u g Laporte-forbidden transitions: g g or u u g stands for gerade – compound with a center of symmetry u stands for ungerade – compound without a center of symmetry Selection rules can be relaxed due to: vibronic coupling (interaction between electron and vibrational modes) spin-orbit coupling geometry relaxation during transition • Symmetry-forbidden transitions – Transitions between states of the same parity are forbidden – Particularly important for centro-symmetric molecules (ethene) – Relaxed by coupling of electronic transitions to vibrational transitions (vibronic coupling) electronic transition Laporte allowed (charge transfer) Laporte forbidden (d-d transition) spin allowed; noncentrosymmetiric spin allowed; centrosymmetric spin forbidden Ferdowsi University of Mashhad e 10000 (1000—50000) 100—200 (200—250) 5—100 (20—100) 0.01—1 (< 1) 15 ADVANCED INORGANIC CHEMISTRY ترم طیفی selection rules The Selection rules for electronic transitions Charge-transfer band – Laporte and spin allowed – very intense 3A a 2g →1Eg Laporte and spin forbidden – very weak a, b, and c, Laporte forbidden, spin allowed, intermediate intensity b [Ni(H2O)6]2+ 3A c 2g →3T2g ADVANCED INORGANIC CHEMISTRY ADVANCED INORGANIC CHEMISTRY ترم طیفی [CoCl4]2- [Co(H2O)6]2+ [Mn(H2O)6]2+ Ferdowsi University of Mashhad 18 d-d transition crystal field splitting 5d > 4d > 3d 19 ADVANCED INORGANIC CHEMISTRY Do size and charge of the metal ion and ligands 4d metal ~50% larger than 3d metal 5d metal ~25% larger than 4d metal d-d transition crystal field splitting ADVANCED INORGANIC CHEMISTRY crystal field stabilization energy (CFSE) spin-pairing energy high-spin/low spin configuration d4 ~ d7 d4 20 tetrahedron octahedron elongated octahedron Ferdowsi University of Mashhad square planar 21 ADVANCED INORGANIC CHEMISTRY Dt = 4/9 Do ترم طیفی Tetrahedral ADVANCED INORGANIC CHEMISTRY ترم طیفی 22 Ferdowsi University of Mashhad ADVANCED INORGANIC CHEMISTRY ترم طیفی 23 Ferdowsi University of Mashhad Crystal Field Splitting Energy Forming Complex ADVANCED INORGANIC CHEMISTRY Crystal Field Theory An energy diagram of the orbitals shows all five d orbitals are higher in energy in the forming complex than in the free metal ion, because of the repulsions from the approaching ligands Ligand field theory combines an electrostatic model of metal-ligand interactions (crystal field theory) and a covalent model (molecular orbital theory). 24 OH TD Octahedral 3d Complexes Δo ≈ P (pairing energy) Both low-spin (Δo ≤ P) and high-spin (P ≥ Δo ) complexes are found Tetrahedral Complexes ΔTd = 4/9 Δo hence P >> ΔTd and tetrahedral complexes are always high spin ELECTRONIC STRUCTURE OF HIGH-SPIN AND LOW-SPIN OH COMPLEXES NOTE: SOME FACTORS INFLUENCING THE MAGNITUDE OF Δ-SPLITTING Oxidation State Δo (M3+) > Δo(M2+) e.g. Δo for Fe(III) > Fe(II). The higher oxidation state is likely to be low-spin 5d > 4d >3d e.g. Os(II) > Ru(II) > Fe(II) All 5d and 4d complexes are low-spin. *Crystal Field Splitting Energy - The d orbital energies are “split” with the two dx2-y2 and dz2 orbitals (eg orbital set) higher in energy than the dxy, dxz, and dyz orbitals (t2g orbital set) *The energies of the d orbitals in different environments determines the magnetic and electronic spectral properties of transition metal complexes. ADVANCED INORGANIC CHEMISTRY Crystal Field Theory *Strong-field ligands, such as CN- lead to larger splitting energy *Weak-field ligands such as H2O lead to smaller splitting energy 30 Explaining the Colors of Transition Metals Diversity in colors is determined by the energy difference (D) between the t2g and eg orbital sets in complex ions When the ions absorbs light in the visible range, electrons move from the lower energy t2g level to the higher eg level, i.e., they are “excited” and jump to a higher energy level ADVANCED INORGANIC CHEMISTRY Crystal Field Theory D E electron = Ephoton = hv = hc/l 11/21/2012 The substance has a “color” because only certain wavelengths of the incoming white light are absorbed 31 ADVANCED INORGANIC CHEMISTRY Crystal Field Theory Example – Consider the [Ti(H2O)6]3+ ion – Purple in aqueous solution Hydrated Ti3+ is a d1 ion, with the d electron in one of the three lower energy t2g orbitals The energy difference (DA) between the t2g and eg orbitals corresponds to the energy of photons spanning the green and yellow range These colors are absorbed and the electron jumps to one of the eg orbitals Red, blue, and violet light are transmitted as purple 32 For a given “ligand”, the color depends on the oxidation state of the metal ion – the number of “d” orbital electrons available A solution of [V(H2O)6]2+ ion is violet A solution of [V(H2O)6]3+ ion is yellow For a given “metal”, the color depends on the ligand [Cr(NH3)6]3+ (yellow-orange) ADVANCED INORGANIC CHEMISTRY Crystal Field Theory [Cr(NH3)5]2+ (Purple) Even a single ligand is enough to change the color 33 ADVANCED INORGANIC CHEMISTRY Crystal Field Theory Spectrochemical Series The Spectrochemical Series is a ranking of ligands with regard to their ability to split d-orbital energies For a given ligand, the color depends on the oxidation state of the metal ion For a given metal ion, the color depends on the ligand As the crystal field strength of the ligand increases, the splitting energy (D) increases (shorter wavelengths of light must be absorbed to excite the electrons 34 MAGNETIC PROPERTIES OF TRANSITION METAL COMPLEXES The splitting of energy levels influence magnetic properties Affects the number of unpaired electrons in the metal ion “d” orbitals According to Hund’s rules, electrons occupy orbitals one at a time as long as orbitals of “equal energy” are available When “all” lower energy orbitals are “half-filled (all +½ spin state)”, the next electron can Enter a half-filled orbital and pair up (with a –½ spin state electron) by overcoming a repulsive pairing energy (Epairing) or Enter an empty, higher energy orbital by overcoming the crystal field splitting energy (D) The relative sizes of Epairing and (D) determine the occupancy of the d orbitals ADVANCED INORGANIC CHEMISTRY 36 MAGNETIC PROPERTIES OF TRANSITION METAL COMPLEXES ADVANCED INORGANIC CHEMISTRY The occupancy of “d” orbitals, in turn, determines the number of unpaired electrons, thus, the paramagnetic behavior of the ion Ex. Mn2+ ion ([Ar] 3d5) has 5 unpaired electrons in 3d orbitals of equal energy In an octahedral field of ligands, the orbital energies split The orbital occupancy is affected in two ways: Weak-Field ligands (low D) and High-Spin complexes Strong-Field ligands (high D) and Low-Spin complexes (from spectrochemical series) 37 Explanation of Magnetic Properties Weak-Field ligands and High-Spin complexes Ex. A weak-field ligand, such as H2O, has a “small” crystal field splitting energy (D) It takes less energy for “d” electrons to move to the “eg” set (remaining unpaired) rather than pairing up in the “t2g” set with its higher repulsive pairing energy (Epairing) Thus, the number of unpaired electrons in a weak-field ligand complex is the same as in the free ion Weak-Field Ligands create high-spin complexes, those with a maximum of unpaired electrons Generally Paramagnetic [Mn(H2O)6]2+ Mn2+ ([Ar] 3d5) ADVANCED INORGANIC CHEMISTRY Crystal Field Theory 38 ADVANCED INORGANIC CHEMISTRY TRANS ITION ELEME NTS & THEIR COOR DINATI ON COMP OUNDS Crystal Field Theory Explanation of Magnetic Properties Strong-Field Ligands and Low-Spin Complexes Ex. [Mn(CN)6]4 Strong-Field Ligands, such CN-, cause large crystal field splitting of the d-orbital energies, i.e., higher (D) (D) is larger than (Epairing) Thus, it takes less energy to pair up in the “t2g“ set than would be required to move up to the “eg” set The number of unpaired electrons in a Strong-Field Ligand complex is less than in the free ion Strong-Field ligands create low-spin complexes, i.e., those with fewer unpaired electrons Generally Diamagnetic Fewer unpaired electrons 39 Crystal Field Theory Explaining Magnetic Properties Orbital diagrams for the d1 through d9 ions in octahedral complexes show that both high-spin and low-spin options are possible only for: d4 d5 d6 d7 ions With three “lower” energy t2g orbitals available, the d1, d2, d3 ions always form high-spin (unpaired) complexes because there is no need to pair up Similarly, d8 & d9 ions always form high-spin complexes because the 3 orbital t2g set is filled with 6 electrons (3 pairs) The two t2g orbitals must have either two d8 or one d9 unpaired electron 11/21/2012 40 Explaining Magnetic Properties high spin: weak-field ligand 11/21/2012 low spin: strong-field ligand high spin: weak-field ligand low spin: strong-field ligand ADVANCED INORGANIC CHEMISTRY Crystal Field Theory 41 Magnetic moments of high-spin and low-spin states d4-d7 d4 d5 d6 d7 High Spin P > D n=4 s = 4.90 n=5 s = 5.92 n=4 s = 4.90 * n=3 s = 3.87 * Low Spin D > P n=2 s = 2.83 * n=1 s = 1.73 * n=0 s =0 n=1 s = 1.73 * Some additional orbital contribution to magnetic moment expected Account for the magnetic moments of the following complexes [V(H2O)6]Cl3 = 3.10 [Co(NH3)6]Br2 = 4.55 K4[Fe(CN)6] =0 11/21/2012 44 PRACTICE PROBLEM ADVANCED INORGANIC CHEMISTRY Iron(II) forms an essential complex in hemoglobin For each of the two octahedral complex ions [Fe(H2O)6]2+ [Fe(CN)6]4Draw an orbital splitting diagram, predict the number of unpaired electrons, and identify the ion as low-spin or high spin Ans: Fe2+ has the [Ar] 3d6 configuration H2O produces smaller crystal field splitting (D) than CNThe [Fe(H2O)6]2+ has 4 unpaired electrons (high spin) The [Fe(CN)6]4- has no unpaired electrons (low spin) Four electron groups about the central atom Four ligands around a metal ion also cause d-orbital splitting, but the magnitude and pattern of the splitting depend on the whether the ligands are in a “tetrahedral” or “square planar” arrangement Tetrahedral – AX4 Octahedral – AX4E2 (2 ligands along “z” axis removed) Splitting of d-orbital energies by a tetrahedral field of ligands Splitting of d-orbital energies by a square planar field of ligands. ADVANCED INORGANIC CHEMISTRY Crystal Field Theory 45 ADVANCED INORGANIC CHEMISTRY Crystal Field Theory (Splitting) Tetrahedral Complexes Ligands approach corners of a tetrahedron None of the five metal ion “d” orbitals is directly in the path of the approaching ligands Minimal repulsions arise if ligands approach the dxy, dyz, and dyz orbitals closer than if they approach the dx2-y2 and dz2 orbitals (opposite of octahedral case) Thus, the dxy, dyz, and dyz orbitals experience more electron repulsion and become higher energy Splitting energy of d-orbital energies is “less” in a tetrahedral complex than in an octahedral complex Dtetrahedral < Doctahedral Only high-spin tetrahedral complexes are known because the magnitude of (D) is small (weak) 46 ADVANCED INORGANIC CHEMISTRY Crystal Field Theory (Splitting) Square Planar Complexes Consider an Ocatahedral geometry with the two z axis ligands removed, no z-axis interactions take place Thus, the dz2, dxz an dyz orbital energies decrease The two ‘d” orbitals in the xy plane (dxy, dx2-y2) interact most strongly with the approaching ligands The (dxy, dx2-y2) orbital has its lobes directly on the x,y axis and thus has a higher energy than the dxy orbital Square Planar complexes are generally strong field – low spin and generally diamagnetic D8 metals ions such as [PdCl4]2- have 4 pairs of the electrons filling the lowest energy levels and are thus, “diamagentic” 47























































![Chem 400 Chem 340 Inorg Review [AR].S17](http://s1.studyres.com/store/data/000220292_1-82084c4723d43bb722b21295b237196f-150x150.png)
