* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Morphology: Allograft Heart Valves
Survey
Document related concepts
Transcript
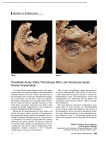
22 Morphology: Allograft Heart Valves Stephen L. Hilbert, Frederick J. Schöen, and Victor J. Ferrans Aortic and Pulmonary Valves General Morphologic Features Aortic and pulmonary valves are referred to collectively as semilunar valves. The normal aortic valve is non-obstructive when open, competent when closed, non-thrombogenic, noninjurious to blood cells, durable, resistant to infection and, capable of continuously remodeling its extracellular matrix and repairing itself when injured. The dilated pockets of aortic root behind the valve cusps bulge with each systolic ejection of blood and are called sinuses of Valsalva.1 Normally, the three aortic valve cusps fold back into their respective sinuses of Valsalva when the left ventricular (LV) pressure exceeds that in the aortic root (in ventricular systole).When aortic root pressure exceeds that in the LV cavity, the cusps fall back across the outflow tract. Prolapse into the LV is prevented by their concave semilunar shape and coaptation of cuspal free edges (in ventricular diastole). Aortic valve function normally relies on its 3 cusps, the annular fibrous tissue, and aortic root/sinuses of Valsalva. The aortic valve cusps attach to the aortic wall in a crescentic fashion, ascending to sites where adjacent cusps are separated by only a small distance (commissures) and descending to the trough of each cusp.1 The three commissures are spaced circumferentially approximately 120° apart and occupy the three points of a triradiate crown. The aortic valve cusps are named for their rela- 200 tionship to the coronary artery ostia; thus, a normal valve has right, left and non-coronary cusps. The structure of the pulmonary valve and surrounding tissues is similar to that of the aortic valve, except for a thinner structure, lack of well-developed sinuses behind the cusps and absence of coronary arterial orifices. The three aortic cusps have a similar shape, resembling that of a half-moon (frequently referred to as semilunar cusps), but are usually unequal in size. The thin, crescentic region of each cusp between its free edge and closing edge, termed the lunula, defines the coaptive region of the cusps. During valve closure, the individual halves of the lunulas of one cusp contact the corresponding regions of both adjacent cusps, thereby effecting a competent seal. A fibrous mound, known as the nodule of Arantius (nodulus Arantii), is located on the ventricular surface, in the middle of the free edge of each cusp.1 Visible semilunar ridges 2 to 3 mm from the cuspal free edge define the lower edge of the lunula and rise to meet the nodule of Arantius. Coaptation of the three nodules ensures complete central closure of the valve orifice during ventricular diastole. Since the cross-sectional area of the aortic root is smaller than the total surface area of the cusps, normal aortic valve cusps overlap as much as 40% of their area in the closed position. Although it is common to have fenestrations (holes) near the free edges as a developmental or degenerative abnormality, this generally has no functional significance, since the lunular tissue does not contribute to separating aortic 22. Morphology: Allograft Heart Valves 201 Figure 22.1. Light micrograph illustrating the characteristic histologic appearance of a porcine aortic valve. Three distinct regions are present: the ventricularis (v), the spongiosa (s), and the fibrosa (f). H&E stain. ¥ 100. from ventricular blood during diastole. In contrast, fenestrations below the lunula cause incompetence and they suggest a previous or active infection. The aortic and pulmonary valves have a well defined histologic structure1 and lack intrinsic blood vessels, since they are sufficiently thin to receive nutrients by diffusion from surrounding blood. The presence of nerve fibers and nerve terminals have recently been demonstrated in aortic valve cusps, although the functional significance of this finding is unknown.2 The cusps of the semilunar valves consist of three distinct histologic layers (from the inflow surface): the ventricularis, the spongiosa and the fibrosa (Figure 22.1). These layers are similar in distribution in all semilunar valves; however, the pulmonary valve is thinner and more delicate in appearance.1 Ultrastructural studies of the pulmonary valve have been limited.3 Thus, the description which follows is based primarily on studies of the aortic valve in humans and in various animal species.4–13 Among the latter, porcine aortic valves have been extensively studied, due to their use as bioprosthetic heart valves. The only significant morphologic difference between the human and the porcine aortic valve involves the right coronary cusp, which in swine is larger than the other two cusps and contains a “muscle shelf” located in the basal one-third to one-half of the cusp. The “muscle shelf” consists of cardiac myocytes and their vascular supply and represents an extension of the ventricular septal muscle into the basal region of the cusp. This muscle layer also may be present, but to a lesser extent, in the non-coronary cusp.6 Thus, the hemodynamic implications of the presence of a “muscle shelf” (such as an asynchronous, delayed opening of the cusp), a reduced effective orifice area, and the propensity of this region to postimplantation degenerative calcification, are of no concern with the use of human allograft valves. Cellular Components Four major types of cells are present in cardiac valves: 1) endothelial cells; 2) interstitial connective cells; 3) mononuclear cells derived from the blood, and 4) interstitial dendritic cells. The endothelial cells form a continuous monolayer that completely lines the surfaces of the valves and is contiguous with the endothelial cell layer of adjacent regions of the endocardium and/or great vessels. These cells are flattened, have single, centrally located nuclei, contain actinlike and intermediate (10 nm) filaments and are 202 connected by junctional complexes9 that provide a permeability barrier to the diffusion of substances from the blood into the valvular tissue. The interstitial connective tissue cells are present throughout all layers of the valve. Cell counts have demonstrated a relatively uniform distribution of cells within specific histologic regions of porcine aortic valve cusps, (e.g., a mean +/- S.D. of 1760 +/- 312 nuclei/mm2 of tissue section in the spongiosa and 1960≤68 in the fibrosa).14 These interstitial cells usually are referred to as “valvular fibroblasts”, even though they actually show a spectrum of morphologic differentiation.10–12 This spectrum includes: 1) clearly fibroblastic cells (with relatively extensively developed rough-surfaced endoplasmic reticulum); 2) intermediate forms, such as myofibroblasts (with less abundant endoplasmic reticulum, more developed actinlike filaments and peripherally located dense bodies that serve as attachment sites for the filaments) and 3) typical smooth muscle cells, with few cisterns of endoplasmic reticulum but very abundant actin-like filaments, peripherally located dense bodies and well developed basement membranes. These cells have been shown to form a network in which they are interconnected by gap junctions.12 Small numbers of macrophages and lymphocytes (mostly T- S.L. Hilbert et al. lymphocytes) are also present throughout the various layers of the valves. In addition, interstitial dendritic cells also have been demonstrated to be present in heart valves. These cells are thin, uninucleated and have very slender, elongated cytoplasmic processes, but lack basement membranes and actin filaments. They are similar to cells of this type that are present throughout a variety of other tissues, including the heart.13 By the use of immunohistochemical techniques, they can be identified specifically and distinguished from smooth muscle cells and other types of connective tissue cells.15,15a These cells are presumed to function, as they do in other tissues, in the presentation and processing of antigens. The ventricularis is an extension of the ventricular endocardium. It is subjacent to the spongiosa and is in direct contact with the endothelial cell layer lining the inflow surface of the cusp. The ventricularis contains connective tissue cells (as described above), multidirectionally oriented collagen fibers and an extensive network of elastic fibers. The most prominent elastic fibers are oriented perpendicular to the free edge of the cusp (Figure 22.2). The ventricularis is thickened in the region of cuspal coaptation at the nodulus Arantii in the aortic valve (nodulus Morgagni in the pul- Figure 22.2. Histologic section of a porcine aortic valve demonstrating abundant, radially oriented elastic fibers (arrowhead) within the ventricularis. The inflow and outflow surfaces of the leaflet are lined by endothelial cells. Movat pentachrome stain. ¥ 200. 22. Morphology: Allograft Heart Valves 203 Figure 22.3. Transmission electron micrograph of the outflow surface of a native porcine aortic valve. Note the presence of an intact endothelial cell layer, a cell-cell junctional complex (arrowhead) and reduplication of the basement membrane (arrow). Fibroblasts, collagen fibrils and elastic fibers (stained black) are seen in the fibrosa. Kajikawa stain. ¥ 9,000. monary valve).1 As a practical note, the ultrastructural study of cardiac valves involves a number of unusually difficult problems, particularly the proper identification of the inflow and outflow regions of the tissue specimen being examined. For this purpose, an elastic fiber stain (Kajikawa) (Figure 22.3) is preferable to routine electron microscopic stains, since it facilitates the identification of the ventricularis.6 The central layer of the cusp is referred to as the spongiosa.This layer is histologically similar in semilunar and in atrioventricular valves and is composed of loosely arranged collagen fibers, fibroblasts and other types of connective tissue cells embedded in an extracellular matrix rich in proteoglycans. The spongiosa is particularly prominent in the basal aspect of the cusp and does not extend to the free edge, which consists of only the fibrosa and the ventricularis. The fibrosa is composed of fibroblasts, other types of connective tissue cells and dense collagen bundles, which serve as the major structural component of the outflow region of the cusp. The collagen bundles approaching the commissures are arranged into densely packed cords, which are integrated into the valvular ring. A few connective tissue cells, including elongated fibroblasts and myofibroblasts,10–12 and small numbers of elastic fibers are present in the fibrosa. Elastic fibers are prominently seen in the basal region of the cusp near the outflow surface and appear as a distinct histologic layer, referred to as the arterialis. A single layer of endothelial cells lines the outflow surface of the cusp. Reduplication of the endothelial basement membrane is a unique ultrastructural marker that serves to identify the outflow surface of the porcine aortic valve cusp (Figure 22.3).6 Morphologic studies of the aortic valve surface have provided insights into the effects of the physical forces (e.g., pressure, tension) applied to the valvular tissue at the time of histologic fixation.4 The inflow surface is much smoother than the outflow surface, demonstrating radially oriented fine striations that correspond to the elastic fibers present in the ventricularis. The outflow surface has a corrugated appearance, due to the presence of coarse, circumferentially arranged collagen bundles in the fibrosa (Figure 22.4). These bundles consist of densely packed collagen fibrils, which appear wavy or crimped when in the relaxed state. As increasing pressure is 204 S.L. Hilbert et al. Figure 22.4. Scanning electron micrograph of the surface of a cryopreserved pulmonary valve allograft, demonstrating the corrugated appearance produced by the underlying collagen bundles and the marked retention of collagen crimp. ¥ 750. applied to the valvular tissue, the collagen fibrils become elongated or straightened, with consequent loss of crimp (Figure 22.5). Polarized light microscopy is particularly well suited for the assessment of the extent of collagen crimp (Figure 22.6). It is noteworthy that pressure gradients as small as 2 mm Hg are sufficient to significantly alter the magnitude of collagen crimp (Figure 22.7).16,17 Extracellular Matrix Cardiac valves are primarily composed of extracellular matrix components of connective Figure 22.5. Transmission electron micrograph demonstrating the loss of collagen crimp, as shown by collagen fibril elongation or straightening. High pressure-fixed porcine aortic valve. ¥ 2,900. 22. Morphology: Allograft Heart Valves 205 Figure 22.6. Polarized light micrographs depicting the morphologic appearance of the collagen bundles and collagen cords of a porcine aortic valve. (A) Characteristic birefringent banding pattern of collagen bundles in which crimp is retained. (B) Loss of collagen crimp (straightening) resulting from tissue elongation by the application of a 100 gram load to the leaflet. ¥ 100. tissue. Maintained by the interstitial cells, these components consist mainly of collagen fibrils, elastic fibers and proteoglycans, which represent the bulk of the physical mass of the cusp. As described above, the histologic organization of these components into three distinct layers Figure 22.7. Histologic section illustrating the effects of pressure fixation on the morphologic appearance of a porcine aortic valve. Note the presence of three distinct histologic regions (ventricularis, spongiosa, fibrosa); however, due to the loss of collagen crimp, the collagen bundles have become elongated, resulting in the overall linear appearance of the valvular tissue. Compare with Figure 6.4. Toluidine blue stain. ¥ 200. determines the unique biomechanical properties and resultant functional characteristics of cardiac valves.9,18–22 Type I and Type III collagen are the most abundant extracellular proteins in cardiac valves; however, Type V collagen is also present as a minor component.23 Recent studies 206 S.L. Hilbert et al. characterizing the types of collagen in cartilage have demonstrated that collagens may be present as either homogeneous fibrils or as mixed fibrils.24 Similar studies of cardiac valve collagens have not been made. The functional effects of proteoglycancollagen interactions may involve the regulation of extracellular polymerization of collagen fibrils.25,26 Domains of high positive charge density are located within the collagen fibril at sites of overlap of adjacent collagen molecules (i.e., carboxyl- and amino-terminal end of the polypeptide chain; staggered overlap region). These sites are involved in the formation of intermolecular cross-links in the collagen fibril. The extent of the cross-linking of the collagen fibrils may be regulated by the presence of proteoglycan glucosaminoglycan side chains and at these cationic sites.25,26 These concepts are of theoretical and practical significance concerning the direction of future research on the viability of cells in cryopre- served allografts and on the mechanisms of renewal of the extracellular matrix of allograft valves. In addition to the various morphologic characteristics of allograft valves, a variety of gender and age-related anatomic and histologic changes occur in human aortic valves. The following changes are most frequently observed in valves harvested from male donors: 1) degeneration of collagen fibers; 2) a fibroelastic “spur” along the coaptation surface; 3) a decrease in the number of cuspal fibroblasts and in the amount of proteoglycans; 4) accumulation of extracellular lipid (Figure 22.8) and 5) calcific deposits (Figure 22.9).27–29 Because of the prevalence of these changes, current donor criteria for allograft heart valves include an age restriction and a negative medical history of previous cardiac surgery, uncontrolled hypertension, functional cardiac murmurs, rheumatic fever and malignant, autoimmune or vascular diseases.29 Figure 22.8. Explanted aortic valve demonstrating lipid accumulation, collagen fiber degeneration and the loss of typical valvular histologic features. The cleft-shaped inclusions indicate that cholesterol esters were present in the valvular tissue. H&E stain. ¥ 200. 22. Morphology: Allograft Heart Valves 207 Figure 22.9. Histologic section of a calcific nodule within the spongiosa of a porcine aortic valvular bioprothesis. This nodule has resulted in deformation and disorganization of the collagen bundles in the fibrosa. Note the loss of the histologic layering of the valve and the insudation of plasma proteins (arrow) and lipids (arrowhead). Toluidine blue stain. Glycol methacrylate. ¥ 100. Mitral Valve semilunar valves, this layer dips into the insertion sites of the chordae tendineae. The fibrosa contains fibroblasts and other types of connective tissue cells, as well as a continuous layer of crimped collagen bundles that are oriented parallel (circumferential) to the ventricular surface and continue into the chordae tendineae. The latter structures consist of an outer layer lined by endothelial cells and an inner core. The outer layer has a subendothelial region containing a few collagen and elastic fibers. As mentioned above, the inner core is a continuation of the valvular fibrosa and consists of longitudinally oriented, crimped collagen bundles. Fibroblasts and myofibroblasts are interspersed between the collagen bundles in these areas. The ventricularis represents a continuation of the ventricular endocardium. It is covered by an endothelial cell layer with a subendothelial region rich in proteoglycans and elastic fibers. The elastic fibers in the ventricularis of the posterior cusp are shorter than those in the auricularis. The ventricularis of the anterior cusp, in General Morphologic Features The anterior mitral valve leaflet is significantly larger than the posterior leaflet; in addition, there are a few histologic differences.1 The histologic appearance of the mitral valve leaflet is similar to that previously described for the semilunar valve cusps. The auricularis of the mitral valve consists of an elastic lamina with interspersed collagen fibers and smooth muscle cells lying beneath a continuous layer of endothelial cells. The auricularis may comprise as much as 20% of the leaflet structure and represents a continuation of the atrial endocardium, extending over approximately 60% of the leaflet surface. At the free edge of the leaflet, all the histologic regions merge, and the elastic lamellae are less superficial and are covered by a layer of connective tissue containing collagen fibers and few cells. The spongiosa is histologically similar to that of aortic and pulmonary valves. In contrast to 208 S.L. Hilbert et al. contrast to that of the posterior cusp, is a continuation of the subaortic endocardium and can be readily identified by the presence of dense elastic fibers, which may be more prominent than those in the auricularis. References 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Gross L, Kugel MA. Topographic anatomy and histology of the valves in the human heart. Am J Pathol 1931;7:445–473. Marron K, Yacoub MH, Polak JM, et al. Innervation of human atrioventricular and arterial valves. Circulation 1996;94:368–375. Kolb R,Pischinger A,Stockinger L.Ultrastuktur der Pulmonalisklappe des meerschweinchens. Beitrag zum Studium der egetative-nervosen Pripheris. Z Mikrosk Anat Forsch 1967;76:184– 211. Clark RE, Finke EH. The morphology of stressed and relaxed human aortic leaflets.Trans Am Soc Artif Intern Organs 1974;20B:437–448. Missirlis YF, Armeniades CD. Ultrastructure of the human aortic valve.Acta Anat (Basel) 1977; 98:199–205. Ferrans VJ, Spray TL, Billingham ME, Roberts WC. Structural changes in glutarraldehydetreated porcine heterografts used as substitute cardiac valves. Transmission and scanning electron microscopic observations in 12 patients. Am J Cardiol 1978;41:1159–1184. Wheeler EE, Gavin JB, Herdson PB. A scanning electron microscopy study of human heart valve allografts. Pathology 1972;4:185–192. Hammon JW, Jr., O’Sullivan MJ, Oury J, Fosburg RG. Allograft cardiac valves. A view through the scanning electron microscope. J Thorac Cardiovasc Surg 1974;68:352–360. Swanson WM, Clarke RE. Dimensions and geometric relationships of the human aortic valve as a function of pressure. Circ Res 1974; 35:871–882. Messier RH, Bass BL, Aly HM. Dual structural and functional phenotypes of the porcine aortic valve interstitial characteristics of the leaflet myofibroblast. J Surg Res 1994;57:1–21. Mulholland DL, Gotlieb AI. Cell biology of valvular interstilial cells. Canadian Journal of Cardiology 1996;12:231–236. Filip DA, Radu A, Simionescu M. Interstitial Cells of the Heart Valves Possess Characteristics Similar to Smooth Muscle Cells. Circulation Research 1986;59:310–319. 13. Gavriel Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992;119:493–501. 14. Schoen FJ, Levy RJ, Nelson AC, et al. Onset and progression of experimental bioprosthetic heart valve calcification. Lab Invest 1985;52: 523–532. 15. Zhang J, Yu ZX, Fuijita S, et al. Interstitial dendritic cells of the rat heart. Quantitative and ultrastructureal changes in experimental myocardial infarction. Circulation 1993;87:909– 920. 15a. Maish M. Hoffman-Kim D, Krueger PM, Harper JJ, Hopkins RA. Tricuspid valve biopsy: A potential source of cardiac myofibroblast cells for tissue engineered cardiac valves. J Heart Valve Dis 2003;12:264–269. 16. Hilbert SL, Barrick MK, Ferrans VJ. Porcine aortic valve bioprosthesis: A morphologic comparison of the effects of fixation pressure. J Biomed Mat Res 1990;24:773–787. 17. Flomenbaum MA, Schoen FJ. Effects of fixation back pressure and antimineralization treatment on the morphology of porcine aortic bioprosthetic valves. J Thorac Cardiovasc Surg 1993;105:154–164. 18. Broom ND, Christie GW. The structure/function relationship of fresh and glutaraldehydefixed aortic valve leaflets. In Cohn LH, Gallucci V (eds). Cardiac Bioprostheses. New York: Yorke 1982:476–491. 19. Missirlis MF, Chong M. Aortic valve mechanics. I. Material properties of native porcine aortic valves. J Bioeng 1978;12:287–300. 20. Williams BT, Bellhouse BJ, Ashton T. Autologous superior vena cava as a material for heart valve replacement. J Thorac Cardiovasc Surg 1973;66:952–958. 21. Brewer RJ, Deck JD, Capati B, Nolan SP. The dynamic aortic root. Its role in aortic valve function. J Thorac Cardiovasc Surg 1976;72: 413–417. 22. Thubrikar MJ, Aouad J, Nolan SP. Comparison of the in vivo and in vitro mechanical properties of aortic valve leaflets. J Thorac Cardiovasc Surg 1986;92:29–36. 23. Bashey RI, Jimenez SA. Collagen in Heart Valves. In Nimni ME (ed). Collagen. Boca Raton, FL: CRC Press 1988:257–273. 24. Scotten LN, Walker DK, Brownlee RT. The in vitro function of 19 mm bioprosthetic heart valves in the aortic position. Life Support Systems 1986;5:145–153. 22. Morphology: Allograft Heart Valves 25. 26. 27. Ruggerri A, Benazza F. Collagen-proteoglycan interactions. In Ruggerri A, Motta PM (eds). Ultrastructure of the Connective Tissue Matrix. Boston: Nijhoff 1984:113–125. Thyberg CJO. Electron microscopy of proteoglycans. In Ruggerri A, Motta PM (eds). Ultrastructure of the Connective Tissue Matrix. Boston: Nijhoff 1984:95–112. Smith JC. The pathology of human aortic valve homografts. Thorax 1967;22:114–138. 209 28. 29. Ross D, Yacoub MH. Homograft replacement of the aortic valve. A critical review. Prog Cardiovasc Dis 1969;11:275–293. Lange PL, Hopkins RA, Brockbank K. Allograft valve banking:Techniques and technology. In Hopkins RA (ed). Cardiac reconstructions with allograft valves. New York: SpringerVerlag 1989:37–64.