* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 5 Thermochemistry
Solar air conditioning wikipedia , lookup
Thermodynamics wikipedia , lookup
Heat transfer wikipedia , lookup
Marcus theory wikipedia , lookup
Electrolysis of water wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Internal energy wikipedia , lookup
George S. Hammond wikipedia , lookup
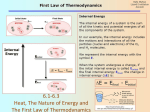
Have you ever wondered how your body regulates its own temperature? The human body has the ability to maintain a constant temperature within the range 96.599°F (35.8-37.2°C) This is controlled by your own thermostat, the hypothalamus gland. This gland controls the rate at which the body metabolizes glucose and other heat related functions C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l) ΔH = -2803 kJ/mol Thermochemistry C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l) ΔH = -2803 kJ/mol • For every mole of glucose you burn, you are burning _____ calories! • This process releases energy, therefore it is exothermic. • The hypothalamus gland controls other mechanisms in the body to help retain or release heat. • Thermochemistry at work!!! Thermochemistry Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Hmwk pg. 188-196, # 7, 12, 15, 19, 21, 23, 29, 33, 41, 45, 51, 53, 59, 61, 67, 71, 77, 83, 97, 103 Chapter 5 Thermochemistry John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall, Inc. Thermochemistry Energy • The ability to do work or transfer heat. Work: Energy used to cause an object that has mass to move. Heat: Energy used to cause the temperature of an object to rise. Thermochemistry Potential Energy Energy an object possesses by virtue of its position or chemical composition. (Chemicals have potential within the bonds) Thermochemistry Kinetic Energy Energy an object possesses by virtue of its motion. 1 KE = mv2 2 Thermochemistry Units of Energy • The SI unit of energy is the joule (J). kg m2 1 J = 1 s2 • An older, non-SI unit is still in widespread use: The calorie (cal). 1 cal = 4.184 J • Calories that we eat are actually kilocalories are abbreviated as Cal Thermochemistry System and Surroundings • The system includes the molecules we want to study (here, the hydrogen and oxygen molecules). • The surroundings are everything else (here, the cylinder and piston). Thermochemistry Work • Energy used to move an object over some distance. • w = F d, where w is work, F is the force, and d is the distance over which the force is exerted. Thermochemistry Heat • Energy can also be transferred as heat. • Heat flows from warmer objects to cooler objects. • Recall, cold is simply a lack of heat. Thermochemistry Transferal of Energy a) The potential energy of this ball of clay is increased when it is moved from the ground to the top of the wall. Thermochemistry Transferal of Energy a) The potential energy of this ball of clay is increased when it is moved from the ground to the top of the wall. b) As the ball falls, its potential energy is converted to kinetic energy. Thermochemistry Transferal of Energy a) The potential energy of this ball of clay is increased when it is moved from the ground to the top of the wall. b) As the ball falls, its potential energy is converted to kinetic energy. c) When it hits the ground, its kinetic energy falls to zero (since it is no longer moving); some of the energy does work on the ball, the rest is dissipated as heat. Thermochemistry First Law of Thermodynamics • Energy is neither created nor destroyed. • In other words, the total energy of the universe is a constant; if the system loses energy, it must be gained by the surroundings, and vice versa. Use Fig. 5.5 Thermochemistry Internal Energy The internal energy of a system is the sum of all kinetic and potential energies of all components of the system; we call it E. Use Fig. 5.5 Thermochemistry Internal Energy By definition, the change in internal energy, E, is the final energy of the system minus the initial energy of the system: E = Efinal − Einitial Use Fig. 5.5 Thermochemistry Changes in Internal Energy • If E > 0, Efinal > Einitial Therefore, the system absorbed energy from the surroundings. This energy change is called endergonic. Thermochemistry Changes in Internal Energy • If E < 0, Efinal < Einitial Therefore, the system released energy to the surroundings. This energy change is called exergonic. Thermochemistry Changes in Internal Energy • When energy is exchanged between the system and the surroundings, it is exchanged as either heat (q) or work (w). • That is, E = q + w. Thermochemistry E, q, w, and Their Signs Thermochemistry Exchange of Heat between System and Surroundings • When heat is absorbed by the system from the surroundings, the process is endothermic. Thermochemistry Exchange of Heat between System and Surroundings When heat is released by the system to the surroundings, the process is exothermic. Thermochemistry State Functions Usually we have no way of knowing the internal energy of a system; finding that value is simply too complex a problem. Thermochemistry State Functions • However, we do know that the internal energy of a system is independent of the path by which the system achieved that state. In the system below, the water could have reached room temperature from either direction. Thermochemistry State Functions • Therefore, internal energy is a state function. • It depends only on the present state of the system, not on the path by which the system arrived at that state. • And so, E depends only on Einitial and Efinal. Thermochemistry State Functions • However, q and w are not state functions. • Whether the battery is shorted out or is discharged by running the fan, its E is the same. But q and w are different in the two cases. Thermochemistry State Functions Yes! Your bank account (the balance is $25 whether you simply deposited $25 or deposited $100 and withdrew $75) No! The distance traveled from Penncrest to K o P mall. The distance will depend on the route taken. Thermochemistry Work When a process occurs in an open container, commonly the only work done is a change in volume of a gas pushing on the surroundings (or being pushed on by the surroundings). Thermochemistry Work We can measure the work done by the gas if the reaction is done in a vessel that has been fitted with a piston. w = −PV Thermochemistry Enthalpy • If a process takes place at constant pressure (as the majority of processes we study do) and the only work done is this pressure-volume work, we can account for heat flow during the process by measuring the enthalpy of the system. • Enthalpy is the internal energy plus the product of pressure and volume: H = E + PV Thermochemistry Enthalpy • When the system changes at constant pressure, the change in enthalpy, H, is H = (E + PV) • This can be written H = E + PV Thermochemistry Enthalpy • Since E = q + w and w = −PV, we can substitute these into the enthalpy expression: H = E + PV H = (q+w) − w H = q • So, at constant pressure the change in enthalpy is the heat gained or lost. Thermochemistry Endothermicity and Exothermicity • A process is endothermic, then, when H is positive. Thermochemistry Endothermicity and Exothermicity • A process is endothermic when H is positive. • A process is exothermic when H is negative. Thermochemistry Endo or Exo? State whether ΔH is positive or negative • • • • • The combustion of fuel in a car engine Salting winter roads Making snow on the ski trails Skiing down the trails Snow-covered trails melt in the spring time. • Placing an ice pack on an injury Thermochemistry • The combustion of fuel in a car engine Heat is leaving, ΔH is negative, exothermic • Salting winter roads Energy between NaCl molecules will leave, ΔH is negative, exothermic • Making snow on the ski trails Heat is leaving, ΔH is negative, exothermic • Skiing down the trails Kinetic energy is dissipated in the snow under the skis in the form of heat, ΔH is negative, exothermic • Snow covered trails melt in the spring time Heat is absorbed, ΔH is positive, endothermic • Placing an ice pack on an injury Heat released by injury is absorbed, ΔH is positive, endothermic Thermochemistry Enthalpies of Reaction The change in enthalpy, H, is the enthalpy of the products minus the enthalpy of the reactants: H = Hproducts − Hreactants Thermochemistry Enthalpies of Reaction This quantity, H, is called the enthalpy of reaction, or the heat of reaction. Thermochemistry The Truth about Enthalpy 1. Enthalpy is an extensive property. 2. H for a reaction in the forward direction is equal in size, but opposite in sign, to H for the reverse reaction. 3. H for a reaction depends on the state of the products and the state of the reactants. Thermochemistry Enthalpy Calculation Sucrose is oxdized to carbon dioxide and water. The enthalpy change can be measured in the laboratory as shown C12H22O11 + 12O2 → 12CO2 + 11H2O ΔH = -5645 kJ What is the enthalpy change for the oxidation of 5.00 g of sugar? q = -82.5 kJ Thermochemistry Calorimetry Since we cannot know the exact enthalpy of the reactants and products, we measure H through calorimetry, the measurement of heat flow. Thermochemistry Heat Capacity and Specific Heat • The amount of energy required to raise the temperature of a substance by 1 K (1C) is its heat capacity. • We define specific heat capacity (or simply specific heat) as the amount of energy required to raise the temperature of 1 g of a substance by 1 K. Thermochemistry Heat Capacity and Specific Heat Specific heat, then, is heat transferred Specific heat = mass temperature change s= q m T Thermochemistry Constant Pressure Calorimetry By carrying out a reaction in aqueous solution in a simple calorimeter such as this one, one can indirectly measure the heat change for the system by measuring the heat change for the water in the calorimeter. Thermochemistry Constant Pressure Calorimetry Because the specific heat for water is well known (4.184 J/g-K), we can measure H for the reaction with this equation: q = m s T Thermochemistry Using Specific Heat Capacity A 88.5-g piece of iron has a temperature of 78.8°C is placed in a beaker containing 244 g of water at 18.8°C. When thermal equilibrium is reached, what is the final temperature? (Assume no heat is lost to warm the beaker or the surroundings.) The specific heat of iron is 0.45 J/g-K. Tfinal = 289 K = 16.2°C Thermochemistry Another Example A 15.5-g piece of chromium, heated to 100.0°C, is dropped into 55.5 g of water at 16.5°C. The final temperature of the metal and water is 18.9°C. What is the specific heat capacity of chromium? (Assume no heat is lost to warm the container or the surroundings.) Thermochemistry smetal = 0.44 J/g·K Bomb Calorimetry Reactions can be carried out in a sealed “bomb,” such as this one, and measure the heat absorbed by the water. The bomb and its contents are defined as the system: qrxn = -Ccal x ΔT Ccal is the heat capacity of the bomb calorimeter. Thermochemistry Bomb Calorimetry • Because the volume in the bomb calorimeter is constant, work cannot occur. What is measured is really the change in internal energy, E, not H. • For most reactions, the difference is very small. Thermochemistry Using a Bomb Calorimeter Methylhydrazine (CH6N2) is commonly used as a liquid rocket fuel. The combustion of methylhydrazine is as follows: 2CH6N2 + 5O2 → 2N2 + 2CO2 + 6H2O When 4.00 g of methylhydrazine is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 25.00°C to 39.50°C. In a separate experiment the heat capacity of the calorimeter is measured to be 7.794 kJ/°C. What is the heat of reaction for the combustion of a mole of CH6N2 in this calorimeter? Was the reaction endo- or exothermic? -1.30 x103 kJ/mol CH6N2 Thermochemistry Hess’s Law H is well known for many reactions, and it is inconvenient to measure H for every reaction in which we are interested. • However, we can estimate H using H values that are published and the properties of enthalpy. Thermochemistry Recall…The Truth about Enthalpy 1. Enthalpy is an extensive property. 2. H for a reaction in the forward direction is equal in size, but opposite in sign, to H for the reverse reaction. 3. H for a reaction depends on the state of the products and the state of the reactants. Thermochemistry Hess’s Law Hess’s law states that “If a reaction is carried out in a series of steps, H for the overall reaction will be equal to the sum of the enthalpy changes for the individual steps.” Thermochemistry Hess’s Law Because H is a state function, the total enthalpy change depends only on the initial state of the reactants and the final state of the products. Thermochemistry Example Calculate ΔH for this reaction 2C (s) + H2 (g) → C2H2 (g) Given the following reactions and their respective enthalpy changes C2H2 (g) + 5/2O2 (g) → 2CO2 (g) + H2O (l) ΔH = -1299.6 kJ C (s) + O2 (g) → CO2 (g) ΔH = -393.5 kJ H2 (g) + 1/2O2 (g) → H2O (l) ΔH = -285.8 kJ 226.8 kJ Thermochemistry You Try This One Suppose you want to know the enthalpy change for the formation of methane, CH4, from solid carbon (as graphite) and hydrogen gas: C(s) + 2H2 (g) → CH4 (g) ΔH = ? The enthalpy change for this reaction cannot be measured in the laboratory because the reaction is very slow. We can, however, measure the enthalpy changes for the combustion of carbon, hydrogen and methane. C (s) + O2 (g) → CO2 (g) ΔH = -393.5 kJ H2 (g) + ½ O2 (g) → H2O (l) ΔH = -285.8 kJ CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l) ΔH = -890.3 kJ Use these energies to obtain ΔH for the Thermochemistry formation of methane. ΔH = -74.8 kJ Calculation of H C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) • Imagine this as occurring in 3 steps: C3H8 (g) 3 C(graphite) + 4 H2 (g) 3 C(graphite) + 3 O2 (g) 3 CO2 (g) 4 H2 (g) + 2 O2 (g) 4 H2O (l) Thermochemistry Calculation of H C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) • Imagine this as occurring in 3 steps: C3H8 (g) 3 C(graphite) + 4 H2 (g) 3 C(graphite) + 3 O2 (g) 3 CO2 (g) 4 H2 (g) + 2 O2 (g) 4 H2O (l) Thermochemistry Calculation of H C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) • Imagine this as occurring in 3 steps: C3H8 (g) 3 C(graphite) + 4 H2 (g) 3 C(graphite) + 3 O2 (g) 3 CO2 (g) 4 H2 (g) + 2 O2 (g) 4 H2O (l) Thermochemistry Calculation of H C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) • The sum of these equations is: C3H8 (g) 3 C(graphite) + 4 H2 (g) 3 C(graphite) + 3 O2 (g) 3 CO2 (g) 4 H2 (g) + 2 O2 (g) 4 H2O (l) C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) Thermochemistry Enthalpies of Formation An enthalpy of formation, Hf, is defined as the enthalpy change for the reaction in which a compound is made from its constituent elements in their elemental forms. Thermochemistry Standard Enthalpies of Formation Standard enthalpies of formation, Hf, are measured under standard conditions (25°C and 1.00 atm pressure) and represent the change in enthalpy associated with the reaction that forms 1 mole of the compound from its elements, with all substances in their standard states. If the element exists in more than one form, the most stable form of the element is used for the formation reaction. Thermochemistry Calculation of H C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) H = = = = [3(-393.5 kJ) + 4(-285.8 kJ)] - [1(-103.85 kJ) + 5(0 kJ)] [(-1180.5 kJ) + (-1143.2 kJ)] - [(-103.85 kJ) + (0 kJ)] (-2323.7 kJ) - (-103.85 kJ) Notice, that by -2219.9 kJ definition, the standard enthalpy of formation of the most stable form of any element is zero, and therefore, the standard enthalpy Thermochemistry of O2 is zero. Calculation of H We can use Hess’s law in this way: H = nHf(products) - mHf(reactants) where n and m are the stoichiometric coefficients. Thermochemistry Standard Enthalpies of Formation Example: Write the equation corresponding to the standard enthalpy of formation of acetylene (C2H2) gas. C(graphite) + H2(g) → C2H2 (g) Write the equation corresponding to the standard enthalpy of formation of solid silver chloride. Ag(s) + ½Cl2(g) → AgCl(s) Thermochemistry Use the Formula Given the following standard enthalpy of reaction, use the standard enthalpies of formation in the table to calculate the standard enthalpy of formation of CuO(s) CuO(s) + H2(g) → Cu(s) + H2O(l) ΔH° = -129.7 kJ Thermochemistry Energy in Foods Most of the fuel in the food we eat comes from carbohydrates and fats. The energy content in foods in reported in Calories and is determined with the use of a bomb calorimeter. Thermochemistry Fuels The vast majority of the energy consumed in this country comes from fossil fuels. Thermochemistry Thermochemistry is Clearly Important to Our Everyday Lives • Personal Nutrition – nutritional value labels • Creation of alternative fuel sources • Addressing Global Warming Thermochemistry Chapter Summary • • • • • • Thermodynamics is the study of energy and its transformations. All chemical changes involve a transfer of energy, be it into the reaction or out of the reaction. Transformed energy in a chemical reaction comes from or forms chemical bonds and is exchanged with the surroundings as heat and/or work. When a gas is produced or consumed in a chemical reaction at constant pressure, we call the energy change enthalpy. All substances have a characteristic enthalpy. Calorimeters and bomb calorimeters are devices that allow us to measure the enthalpy change of a chemical reaction by measuring the temperature change associated with such a chemical reaction. Because enthalpy is a state function, the enthalpy of a reaction depends only on the final and initial states of the system and therefore, the enthalpy of a process is the same whether a reaction is carried out in one or in multi-steps. Hess’s Law allows us the calculate the unknown enthalpy of a reaction from a series of know enthalpies of reactions. Thermochemistry