* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download results
Survey
Document related concepts
Transcript
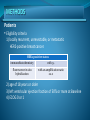
Journal conference Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer José Baselga, M.D., Ph.D., Javier Cortés, M.D., Sung-Bae Kim, M.D., Seock-Ah Im, M.D., Roberto Hegg, M.D.,Young-Hyuck Im, M.D., Laslo Roman, M.D., José Luiz Pedrini, M.D., Tadeusz Pienkowski, M.D.,Adam Knott, Ph.D., Emma Clark, M.Sc., Mark C. Benyunes, M.D., Graham Ross, F.F.P.M., Sandra M. Swain, M.D., for the CLEOPATRA Study Group N Engl J Med 2012;366:109-19. R2. Yujin Um / Prof. Sunkyung Beak BACKGROUND 20% of all breast cancers gene amplification, Overexpression Treatment with Trastuzumab (anti-HER2 humanized monoclonal antibody) + chemotherapy of HER2 (human epidermal growth factor receptor ) more aggressive phenotype a poor prognosis improves progression-free and overall survival among patients with HER2positive metastatic breast cancer N Engl J Med 2007;357:39-51. Figure 1 . Signal Transduction by the HER Family and Potential Mechanisms of Action of Trastuzumab BACKGROUND • Trastuzumab - binds to subdomain IV of the HER2 extracellular domain - antitumor effects by blocking HER2 cleavage stimulating antibody-dependent, cell-mediated cytotoxicity inhibiting ligand-independent, HER2- mediated mitogenic signaling. BACKGROUND HER2-positive metastatic breast cancer disease progresses => the need for new targeted therapies for advanced disease. BACKGROUND • Pertuzumab : Humanized monoclonal antibody : binds HER2 at a different epitope of the HER2 extracellular domain (subdomain II) : prevents HER2 from dimerizing with other ligand-activated HER receptors, most notably HER3 : stimulates antibody-dependent, cell-mediated cytotoxicity BACKGROUND • pertuzumab and trastuzumab : bind to different HER2 epitopes : complementary mechanisms of action : given together => more comprehensive blockade of HER2 signaling => result in greater antitumor activity than either agent alone in HER2-positive tumor models • pertuzumab–trastuzumab regimen : shown activity in patients with HER2-positive metastatic breast cancer in patients with early breast cancer BACKGROUND Clinical Evaluation of Pertuzumab and Trastuzumab study (CLEOPATRA) : assessed the efficacy and safety pertuzumab + trastuzumab + docetaxel placebo + trastuzumab + docetaxel : as first-line treatment : for patients with HER2-positive metastatic breast cancer. METHODS Study Design • randomized, double-blind, placebocontrolled, phase 3 trial HER2-positive metastatic breast cancer : not received chemotherapy or biologic therapy for their metastatic disease. • primary end point - progression-free Survival : on the basis of the assessment of tumors at an independent review facility • secondary end points : overall survival, progression-free survival METHODS Patients • Eligibility criteria 1) locally recurrent, unresectable, or metastatic HER2-positive breast cancer. HER2-positive status immunohistochemistry with 3+ fluorescence in situ hybridization with an amplification ratio ≥2.0 2) age of 18 years or older 3) left ventricular ejection fraction of 50% or more at Baseline 4) ECOG 0 or 1 METHODS Patients • Exclusion criteria 1) therapy for metastatic breast cancer 2) central nervous system metastases 3) prior exposure to a cumulative dose of doxorubicin (> 360 mg per square meter of body-surface area ) 4) previous decline in the left ventricular ejection fraction to less than 50% during or after prior trastuzumab therapy 5) current uncontrolled medical conditions - limit a patient’s ability to undertake study therapy. METHODS Procedures Trastuzumab loading dose 8 mg per kilogram of body weight maintenance dose 6 mg per kilogram every 3 weeks until disease proression, Docetaxel starting dose 75 mg per square Meter Every 3 weeks maintenance dose increased to 100 mg per square meter if the drug had toxic effects reduce the dose by 25%, Pertuzumab fixed loading dose 840 mg until disease progression 420 mg every 3 weeks RESULTS Study Population Table 1. Baseline Characteristics of the Intention-to-Treat Population control group pertuzumab group RESULTS Study Population Table 1. Baseline Characteristics of the Intention-to-Treat Population RESULTS Progression-free Survival Figure 1 . Progression-free Survival, as Assessed at an Independent Review Facility. RESULTS Progression-free Survival Figure 1 . Progression-free Survival, as Assessed at an Independent Review Facility. RESULTS Key Secondary Efficacy End Points Figure 2. Overall Survival. RESULTS Key Secondary Efficacy End Points Table 2. Overall Response, as Assessed at an Independent Review Facility RESULTS Side-Effect Profile and Cardiac Safety Table 2. Overall Response, as Assessed at an Independent Review Facility CONCLUSIONS The combination of pertuzumab plus trastuzumab plus docetaxel, as compared with placebo plus trastuzumab plus docetaxel, when used as first-line treatment for HER2-positive metastatic breast cancer, significantly prolonged progression-free survival, with no increase in cardiac toxic effects.