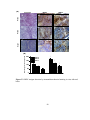
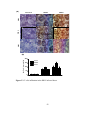
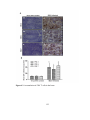
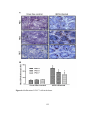
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download persistence, distribution and immunopathogenesis of infectious
Schistosomiasis wikipedia , lookup
Leptospirosis wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Hepatitis C wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Orthohantavirus wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Ebola virus disease wikipedia , lookup
Influenza A virus wikipedia , lookup
West Nile fever wikipedia , lookup
Marburg virus disease wikipedia , lookup
Antiviral drug wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Henipavirus wikipedia , lookup
Hepatitis B wikipedia , lookup
PERSISTENCE, DISTRIBUTION AND IMMUNOPATHOGENESIS OF INFECTIOUS BURSAL DISEASE VIRUS IN CHICKENS DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Abdul Rauf M.Sc. Graduate Program in Veterinary Preventive Medicine The Ohio State University 2011 Dissertation Committee: Dr. Y. M. Saif (Advisor) Dr. Daral J Jackwood Dr. Chang Won Lee Dr. Gireesh Rajashekara Dr. Renukaradhya J. Gourapura Copyrighted by Abdul Rauf 2011 ABSTRACT There have been questions regarding the persistence of infectious bursal disease virus (IBDV) in processed meat destined for export. A series of experiments were designed to determine the persistence, distribution and quantification of IBDV strains in lymphoid and non-lymphoid tissues of specific pathogen free (SPF) chickens and commercial broiler chickens. Five separate experiments were conducted using 2 and 4 weeks old SPF chickens and 2 weeks old commercial broilers having maternally derived antibodies. Four different types of lymphoid and seven different non-lymphoid tissues were sampled from individual chickens in each experiment. Viral RNA (vRNA) was extracted from tissues and subjected to real time RT-PCR. The virus strains were detected the longest in bursal tissues followed by spleen, thymus and bone marrow. In non-lymphoid tissues, both of the strains were detected for the longest period in the caecum followed by liver, kidney, pancreas, lungs, thigh muscles and breast muscles. Upon infection with the standard challenge (STC) strain, a serotype 1 classic virus, the vRNA was detected in bursa of SPF chickens up to 8 weeks post-inoculation (PI) and in commercial broiler chickens the virus was detected up to 6 weeks PI. In addition, we were able to isolate the STC strain up to 4 and 3 weeks PI from the bursal homogenates of SPF and commercial chickens, respectively. The Indiana (IN) strain, a serotype 1 variant virus, vRNA was detected up to ii 5 weeks PI in SPF and up to 3 weeks PI in commercial chickens in bursal tissue. The IN strain was isolated up to 10 days post-inoculation (DPI) from bursal tissues collected from both SPF and commercial chickens. In commercial broilers the STC was detected at DPI 3 in thigh muscles and only one breast muscle sample was RT-PCR positive at DPI 14. The IN strain was not detected in thigh muscles while it was detected in breast muscles up to two weeks post-inoculation (PI) in commercial chickens. Whereas in SPF chickens, the vRNA of STC and IN strains were detected in thigh muscles up to 4 and 2 weeks PI and in breast muscles up to 3 and 2 weeks PI, respectively. This is the first detailed report on the persistence and distribution of classic and variant strains of IBDV in different tissues of SPF and commercial chickens. The study indicated that the persistence and distribution of IBDV in non-lymphoid tissues, specifically, thigh muscles and breast muscles is significantly different between SPF and commercial chickens. Although the virus can persist in the bursa of SPF chickens for 4 weeks, it is very unlikely that the infectious virus will be present in the processed meat. In addition, the RT-PCR results are not sufficient to indicate the presence of the infectious virus. A study was undertaken to better understand the innate immune response against IBDV. In this study 3 weeks old SPF chickens were inoculated intraocularly with STC and Indiana (IN) strains representing either classic IBDV (cIBDV) or variant IBDV (vIBDV), respectively. Bursal lesions, macrophages and T cells infiltration were quantified. We examined the differential expression of virus-induced innate and proinflamatory cytokines, chemokines and toll like receptors (TLRs) and their adaptor molecules. These findings indicate that cIBDV- and vIBDV-infection mediated the differential induction of iii proinflammatory cytokines, innate cytokines and upregulated the expression of TLRs as well as their adaptor molecules. This information will be important in understanding the immune responses against IBDV infection in chickens. In the last part we studied the adaptive immune responses of IBDV in chickens. The IBDV actively replicates in B cells and causes severe bursal damage. Generally, T cells are refractory to infection with IBDV but are known to promote viral clearance. However, the mechanisms of T cell mediated viral clearance are not well understood. In this study, we evaluated the molecular mechanisms of cytotoxic T cell responses in the pathogenesis of IBDV in chickens. Infection of chickens with IBDV was accompanied by the infiltration of CD4+ and CD8+ T cells in the bursa. There was an upregulation in the gene expression of important cytolytic molecules; perforin (PFN), granzyme-A (Gzm-A), DNA repair and apoptotic proteins; high mobility proteins group (HMG) and poly (ADP-ribose) polymerase (PARP) in the bursa of Fabricius (BF), whereas expression of NK (natural killer) lysin was downregulated. Importantly, PFN producing CD4+ and CD8+ T cells were also detected in the bursa of IBDV-infected chickens by immunohistochemistry. The Th1 cytokines, IL-2 and IFN-γ expression was also strongly upregulated, suggesting the activation of T cells. The findings of this study highlight the mechanisms of IBD pathogenesis and the role of cytotoxic T cells in the clearance of virus-infected cells. iv I dedicate this effort to my parents Mr. and Mrs. Abdul Malik Khattak and to all of my Teachers v ACKNOWLEDGEMENTS I wish to express my special gratitude to my advisor Dr. Yehia M. Saif for his support, intellectual guidance, encouragement, and great patience throughout my graduate program. Without his sincere support, guidance, and help, this work would not have been possible. His pleasant, kind and generous personality shaped my thoughts to be an optimistic and look at the brighter aspects of my endeavors. I am forever grateful to him. I wish also to thank my other graduate committee members, Dr. Daral J. Jackwood, Dr. Chang Won Lee, Dr. Gireesh Rajashekara and Dr. Renukaradhya J. Gourapura for their assistance and invaluable advice. I would like to express my sincere appreciation to Dr. Mahesh Khatri for his valuable contribution to my work. I am thankful to Dr. Kwonil Jung and Dr. Alexander Rodriguez-Palaci for their technical support, their invaluable advice and help during my graduate program. I am very grateful to Dr. Juliette Hanson, Gregory Myers, Kingsly Berlin and Todd Root for their support with animal studies. I want to thank my classmates, and colleagues Maria Murgia, and former members of Dr. Y.M Saif lab Dr.Hadi Yassine and Dr. Y. Tang, for their generous support and help. I also want to especially acknowledge Hannah Gehman, Robin Weimer and Megan Strother. I sincerely appreciate what they did for me vi during my time here. Their kind, generous, considerate, and helpful personalities will be remembered forever. Finally, I want to thank my loving parents Mr. and Mrs. Abdul Malik Khattak, my brother Abdul Salam Khattak , I know that I owe their immeasurable debt of gratitude, and I could not compensate it in my life. Without their everlasting and unwavering love, encouragement, faith in me, my dream would not have come true. Very special thanks to my great mom for her understanding, support and encouragement. I would like to express my appreciation for my loving sisters Imrana Malik and Farzana Malik. Finally I owe my greatest appreciation to my wife Naheed, her valuable suggestions made my life much easier than I thought. I thank her very much for taking care of my son Ahsen and daughter Mishall and sparing my time to devote it for my studies. vii VITA B.Sc. (Premedical Group)……………………… 1997 The University of Punjab Lahore, Pakistan B.Ed (Biology and Chemistry)……………….. 1999 AIOU Islamabad, Pakistan M.Sc. (Zoology/Microbiology)……….………. 2000 University of Arid Agriculture, Rawalpindi, Pakistan Research Assistant (Microbiology)……………. 1999-2001, Animal Health institute, National Agriculture Research Center, Islamabad Pakistan Microbiology Research 2001-2006 University of Arid Associate)………………………………………. Agriculture Rawalpindi Pakistan Fulbright Fellow ……………………………… February 2006 to December 2006 FAHRP/OARDC/The Ohio State University, USA Graduate Research Associate (Ph.D. Candidate) 2007-2011 FAHRP/OARDC The Ohio State University, USA Publications 1: Rauf, A., Khatri, M., Murgia, M.V. and Saif, Y.M. (2011) Expression of perforingranzyme pathway genes in the bursa of infectious bursal disease virus-infected chickens. Dev Comp Immunol. In press. viii 2: Functional Invariant NKT Cells in Pig Lungs Regulate the Airway Hyperreactivity: A Potential Animal Model. Renukaradhya, G.J., Manickam, C., Khatri, M., Rauf, A., Li, X., Tsuji, M., Rajashekara, G. and Dwivedi, V. J Clin Immunol. (2010). Published Abstracts 1: Distribution and persistence of infectious bursal disease virus in chickens. Rauf Abdul, Maria V. Murgia, M. Khatri, A. Rodriguez-Palacios, C-W. Lee and Y.M. Saif. North central avian disease conference (61 Annual meeting March 15 & 16 2010) at St. Paul River center St. Paul Minnesota 2: Prevalence of parvoviruses in commercial turkey flocks. Maria V. Murgia, Rauf Abdul, Tang, Y; A. Gingerich, E; C-W. Lee and Y.M. Saif. North central avian disease conference (61 Annual meeting March 15 & 16 2010) at St. Paul River center St. Paul Minnesota 3: Persistence and distribution of infectious bursal disease virus in SPF and commercial broiler chickens. Rauf Abdul, Maria V. Murgia, C-W. Lee, M. Khatri and Y.M Saif. AVMA/AAAP Meeting. Georgia World Congress Center, Atlanta, GA. July31- August 4, 2010: Development of a Real-time PCR test for the detection of Turkey Parvoviruses in fecal samples. Maria V. Murgia, Rauf Abdul and Y.M Saif. AVMA/AAAP Meeting. Georgia World Congress Center, Atlanta, GA. July31- August 4, 2010 viii 5: Viral induced inflammatory cytokine, toll like receptors and cytotoxic T cells components in infectious bursal disease infected chickens. Rauf Abdul, M. Khatri, Maria V. Murgia and Y.M. Saif. Conference for Research workers in animal science at Chicago, December 5 – 7, 2010. Fields of Study Major Field: Veterinary Preventive Medicine Studies in Molecular Virology and Immunology viii TABLE OF CONTENTS PERSISTENCE, DISTRIBUTION AND IMMUNOPATHOGENESIS OF INFECTIOUS BURSAL DISEASE VIRUS IN CHICKENS ............................................ 1 DISSERTATION ................................................................................................................ 1 ABSTRACT ........................................................................................................................ ii TABLE OF CONTENTS ................................................................................................. viii LIST OF TABLES ............................................................................................................. xi CHAPTER 1 ....................................................................................................................... 1 LITERATURE REVIEW ................................................................................................... 1 PERSISTENCE, PATHOGENESIS AND IMMUNOLOGY OF INFECTIOUS BURSAL DISEASE VIRUS ............................................................................................................... 1 CHAPTER 2 ..................................................................................................................... 30 PERSISTENCE AND TISSUE DISTRIBUTION OF INFECTIOUS BURSAL DISEASE VIRUS IN SPF AND COMMERCIAL BROILER CHICKENS ..................................... 30 2.1 SUMMARY ............................................................................................................ 30 2.2 INTRODUCTION ................................................................................................... 32 2.3 MATERIALS AND METHODS ............................................................................ 34 2.4 RESULTS................................................................................................................ 37 viii 2.5 DISCUSSION ......................................................................................................... 40 2.7 REFERENCES ........................................................................................................ 45 CHAPTER 3 ..................................................................................................................... 66 DIFFERENTIAL MODULATION OF CYTOKINES AND TOLL LIKE RECEPTORS EXPRESSION IN THE BURSA OF CHICKENS INFECTED WITH CLASSICAL AND VARIANT STRAINS OF INFECTIOUS BURSAL DISEASE VIRUS. ........................ 66 3.1 SUMMARY..………………………………………………………………………66 3.2 INTRODUCTION ................................................................................................... 67 3.3 MATERIALS AND METHODS ............................................................................ 70 3.4 RESULTS................................................................................................................ 74 3.5 DISCUSSION ......................................................................................................... 76 3.6 ACKNOWLEDGEMENTS .................................................................................... 80 3.7 REFERENCES ........................................................................................................ 81 CHAPTER 4 ................................................................................................................... 101 EXPRESSION OF PERFORIN-GRANZYME PATHWAY GENES IN THE BURSA OF INFECTIOUS BURSAL DISEASE VIRUS-INFECTED CHICKENS .................. 101 4.1 SUMMARY...……………………………………………………………………100 4.2 INTRODUCTION ................................................................................................. 102 4.3 MATERIALS AND METHODS .......................................................................... 104 4.4 RESULTS.............................................................................................................. 108 ix 4.5 DISCUSSION ....................................................................................................... 110 4.6 ACKNOWLEDGEMENTS .................................................................................. 114 4.7 REFERENCES ...................................................................................................... 114 BIBLIOGRAPHY ........................................................................................................... 130 x LIST OF TABLES Table 2.1: Persistence and distribution of IBDV (STC & IN) strains in lymphoid tissues of two-weeks old SPF chickens ........................................................................................ 47 Table 2.2: Persistence and distribution of IBDV (STC & IN) strains in non-lymphoid tissues of two-weeks old SPF chickens ............................................................................ 48 Table 2.3: Persistence and distribution of IBDV (STC&IN) in non-lymphoid tissues of two-weeks old SPF chickens ............................................................................................ 49 Table 2.4: Persistence and distribution of IBDV (STC & IN) strains in lymphoid tissues of four-weeks old SPF chickens ....................................................................................... 50 Table 2.5: Persistence and distribution of IBDV (STC & IN) strains in non-lymphoid tissues of four-weeks old SPF chickens ............................................................................ 51 Table 2.6: Persistence and distribution of IBDV (STC&IN) in non-lymphoid tissues of four-weeks old SPF chickens ............................................................................................ 52 Table 2.7: Persistence and distribution of IBDV strains in lymphoid tissues of two-weeks old in ovo vaccinated broilers chickens ............................................................................ 53 Table 2.8: Persistence and distribution of IBDV strains in non-lymphoid tissues of twoweeks old in ovo vaccinated broilers ................................................................................ 54 Table 2.9: Persistence and distribution of IBDV (STC & IN) strains in non-lymphoid tissues of two-weeks old in ovo vaccinated broilers chickens ......................................... 55 xi Table 2.10: Persistence and distribution of IBDV (STC & IN) strains in lymphoid tissues of four-weeks old in ovo vaccinated broilers chickens ..................................................... 56 Table 2.11: Persistence and distribution of IBDV (STC & IN) strains in non-lymphoid tissues of four-weeks old in ovo vaccinated broilers chickens ......................................... 57 Table 2.12: Persistence and distribution of IBDV (STC & IN) strains in non-lymphoid tissues of four-weeks old in ovo vaccinated broilers ........................................................ 58 Table 2.13: Persistence and distribution of IBDV strains in lymphoid tissues of 2-weeks old broilers having maternal antibodies ............................................................................ 59 Table 2.14: Persistence and distribution of IBDV (STC & IN) strains in non-lymphoid tissues of 2-weeks old broilers having maternal antibodies.............................................. 60 Table 2.15: Persistence and distribution of IBDV (STC & IN) strains in non-lymphoid tissues of 2-weeks old broilers having maternal antibodies.............................................. 61 Table 3.1: Sequence of primers for quantitative real-time RT-PCR analysis…………..85 xii LIST OF FIGURES Figure 2.1. Virus titter decay in tissue samples from chickens singly inoculated with reference strains of infectious bursal disease virus……………………………………. 62 Figure 2.2. Persistence of two strains of infectious bursal disease virus after experimental inoculation in SPF and commercial chickens. .................................................................. 63 Figure 2.3. Log10 viral titers in experimentally inoculated SPF and commercial chickens. ........................................................................................................................................... 64 Figure 2.4. Antibody titer in SPF chickens after inoculation with STC and IN strains…55 Figure 3.1. Histopathological lesions in bursa following IBDV-infection. ..................... 88 Figure 3.2. IBDV antigen detection by immunohistochemical staining in virus-infected bursa. ................................................................................................................................. 90 Figure 3.3. T cells infiltration in the IBDV-infected bursa. ............................................. 92 Figure 3.4. Infiltration of macrophages in the IBDV-infected bursa. .............................. 94 Figure 3.5. Relative gene expression at mRNA level, of innate cytokines mRNA in IBDV-infected bursa. ........................................................................................................ 96 Figure 3.6. Relative gene expression at mRNA level, of proinflamatory cytokines, iNOS and chemokines, mRNA in IBDV-infected bursa. ........................................................... 98 xiii Figure 3.7. Relative gene expression at mRNA level, of Toll like receptors and adaptor molecules mRNA in IBDV-Infected bursa. .................................................................... 100 Figure 4.1. Detection of virus load and IBDV genome in bursa tissue. ........................ 119 Figure 4.2. Gene expression of PFN, Gzm-A and molecules involved in DNA repair and apoptosis in the bursa of IBDV-infected chickens. ........................................................ 121 Figure 4.3. Accumulation of CD8+ T cells in the bursa. ................................................ 123 Figure 4.4. Infiltration of CD4+ T cells in the bursa. ..................................................... 125 Figure 4.5. Detection of PFN producing CD4+ and CD8+ T cells in BF. ...................... 127 Figure 4.6. Expression of Th-1 cytokines in the bursa of IBDV-infected chickens at PIDs 3, 5 and 7. ........................................................................................................................ 129 xiv CHAPTER 1 LITERATURE REVIEW PERSISTENCE, PATHOGENESIS AND IMMUNOLOGY OF INFECTIOUS BURSAL DISEASE VIRUS 1.1 INTRODUCTION Infectious bursal disease (IBD) is a globally distributed immunosuppressive viral disease of young chickens, controlled by vaccination. The disease, also known as Gumboro disease, is endemic in poultry-producing regions, inflicting significant economic losses to the poultry industry worldwide (Flensburg et al., 2002; Saif, 1998; Sanchez et al., 2005). Infectious bursal disease virus (IBDV), the causative agent of IBD, is comprised of two serotypes, designated as serotypes 1 and 2 (McFerran, 1980). Only serotype 1 is pathogenic for the domesticated chicken (Jackwood et al., 1985). Numerous serotype 1 strains, differing in antigenicity have been identified and classified as classic and variant. Chickens are more susceptible to disease when they are 2–6 weeks of age and symptoms are nonspecific, including depression, whitish diarrhea, anorexia, prostration, and death (Chettle et al., 1989). Younger or older chickens may show milder disease symptoms, but all age groups subsequently experience a transient immunosuppression (Dohms and Jaeger, 1988; Kim et al., 1999; Sharma et al., 2000). 1 Classical IBDV has traditionally affected poultry worldwide since the first reported incident from Gumboro, Delaware, USA. Classical strains cause bursal inflammation and severe lymphoid necrosis in infected chicken, resulting in immunodeficiency and moderate mortality from 20-30% in specific pathogen free (SPF) chicken (Lim et al., 1999). Very virulent infectious bursal disease virus (vvIBDV) was isolated in the United States in 2008 and the virus had 80 % sequence homology with classical IBDV strains (Jackwood et al., 2009; Stoute et al., 2009). Variant strains were detected in the US in 1983. These strains were antigenically different from classic strains and caused a rapid and severe bursal atrophy (Vakharia et al., 1994), and in contrast to classical strains produced no clinical signs of illness. Antigenic variants have been recognized by their ability to escape cross-neutralization by antiserum against the classical strains. The Indiana (IN) strain is an antigenic variant strain of IBDV and was first isolated in our laboratory (Ismail et al., 1990). This strain causes severe bursal atrophy at 5 and 10 days post-inoculation in 3-weeks old SPF birds. The IBDV being a non-zoonotic pathogen is ―not regarded as a human food safety issue, nevertheless movement of birds with IBDV infections is a cause for concern because of the possible introduction of new antigenic and pathogenic strains into a geographic area can have a negative economic impact on the chickens grown in that region‖(Jackwood and Sommer-Wagner, 2010). The relative heat resistance of IBDV and its ability to survive 60 or more days in poultry litter (Mandeville et al., 2000), creates some important epidemiological considerations. There have been questions’ regarding the persistence of infectious IBDV in processed meat destined for export. Most of the studies conducted on 2 the persistence of IBDV in tissues are on SPF chickens. Limited data available on the persistence of IBDV in SPF chickens cannot be superimposed on commercial broiler chickens. At present not much is known about the immune responses involved in the pathogenesis of IBDV. The exact cause of clinical disease and death are still unclear and strains of IBDV differ in their virulence. The IBDV infects and destroys actively dividing IgM bearing B cells in the bursa of fabricius (Hirai et al., 1981; Rodenberg et al., 1994). Infection with IBDV compromises both humoral and cellular immunity (Sharma et al., 1989). Immunosuppression induced by IBDV is the primary cause of economic loss associated with the virus (Khatri et al., 2005). Exposure to IBDV results in an acute, selflimiting disease. Under experimental conditions, the acute disease lasts for about a weeks. During this acute phase, replicating virus causes extensive destruction of bursal follicles (Tanimura and Sharma, 1997), resulting in reduction of circulating IgM+ B cells (Hirai et al., 1981; Rodenberg et al., 1994). Macrophages produce proinflamatory cytokines such as IL-6, IL-8, and IL-1β which are important in initiating an inflammatory response at the site of infection. The local inflammatory response recruits other phagocytic and non-phagocytic lymphoid cells important for immunity. Thus, proinflamatory cytokines are a natural response to infection and may be beneficial to the host defense. However, when produced in excess, proinflammatory cytokines may enhance tissue destruction, impede recovery and harm the host (Khatri et al., 2005). 3 Host immune cells use various receptors to perceive viral infections by recognizing pathogen-associated molecular patterns (PAMPs) and subsequently induce an antiviral response. Prominent among these are toll-like receptors (TLRs) (Kawai and Akira, 2006; Sang et al., 2008; Werts et al., 2006). Several TLRs recognize viral PAMPs, of which toll like receptor-3 (TLR3), detects double-stranded RNA (dsRNA) derived from viral replication, whereas single-stranded RNA (ssRNA) are perceived by TLR7 and TLR8 (Sang et al., 2008). The TLR signaling proceeds via two pathways: the myeloid differentiation factor 88 (MyD-88)-mediated pathway and the Toll-interleukin-1 receptor (TIR)-domain-containing adaptor inducing IFN-β (TRIF)-mediated pathway (Kawai and Akira, 2009; Takeuchi and Akira, 2009). TLR3 only activates the TRIF-mediated pathway (Yokota et al., 2010). The three main objectives envisioned for my research are: Persistence and tissue distribution of infectious bursal disease virus in SPF and commercial chickens. Immunopathogenesis and modulation of Toll-like receptor 3 pathway in chickens infected with a classical and a variant strain of infectious bursal disease virus. Expression of perforin-granzyme pathway genes in the bursa of infectious bursal disease virus-infected chickens. 1.2 PERSISTENCE OF IBDV IN CHICKEN TISSUES The bursa and spleen were reported to have substantially higher concentration of the virus as compared to any other tissue. The bursa harvested from chickens at 72 hours PI 4 yielded high virus titers followed by spleen and kidneys. No virus was detected beyond 10 days PI. IBDV was reported to persist in the chicken for a few days but the lesions could be seen for at least 10 weeks, the longest interval evaluated in that study (Winterfield et al., 1972). Chickens were inoculated with an attenuated cell culture adapted virus at one day of age, the virus could be detected in cell culture inoculated with homogenate of BF, spleen, thymus, liver, kidney and the lungs for up to 14 days PI in one experiment and 10 days PI in another. No virus was detected after the levels of neutralizing antibodies became significantly high. Also, the virus was not detected in tissues from birds given the virus at 3 weeks and low virus neutralizing antibody titers were detected, indicating an age resistance to chicken embryo fibroblast cells (CEF) propagated virus or insufficient dose (Skeeles et al., 1979a; Skeeles et al., 1979b). The IBDV was re-isolated most consistently from the bursa and less frequently from thymus, liver, kidneys, lungs and spleen. No virus was isolated from the pancreas (Mackenzie and Spradbrow, 1981). The virus was not detected beyond 11 days PI in commercial chickens when inoculated at 1, 7 or 14 days of age. In birds inoculated at 21 days of age, IBDV was re-isolated up to days only. The precipitating antigen was detected only in the bursa and only at 3rd, 4th and 5th day PI but was not detected in any organ of chicken infected at 21 days of age (Mackenzie and Spradbrow, 1981). When SPF chickens were inoculated with IBDV at 3 weeks of age, the viral RNA was detected by RT/PCR up to 21 days PI, but attempts to isolate infectious virus from bursal homogenates failed (Abdel-Alim and Saif, 2001a). Infectious virus was detected by embryo inoculation up to 7 days PI in the bursa of SPF chickens inoculated at 2 or 3 5 weeks of age, whereas the viral RNA was detected by RT/PCR for up to 28 days PI. In SPF chickens inoculated at 1 day of age, the bursa-derived virus or its RNA was detected 4 2.5 at 7 and 14 days PI when inoculated at a high dose, 10 EID /bird, or at a low dose, 10 50 EID /bird. In commercial 1-day-old broiler chickens, the bursa-derived virus was 50 4 detected at 7 and 14 days PI when inoculated at a high dose, 10 EID50/bird, whereas the 2.5 virus was detected only at 14 days PI when inoculated at a low dose, 10 EID50/bird. In SPF and commercial chickens, vaccinated with a modified live IBDV vaccine, the virus is known to persist in the bursa of SPF chickens up to 3 weeks but maternal antibodies in the commercial chickens rapidly eliminate it from the bursa since no live vaccine virus nor its RNA was detected in commercial broilers vaccinated at 1 day or 2 weeks of age (Abdel-Alim and Saif, 2001a). In an another study it was documented that variant IBDV was detected in virus-inoculated commercial broilers for up to 6 weeks and infectious virus was recovered from all organs at 4 weeks PI (Elankumaran et al., 2002). This was the first report that mentions that IBDV can be detected up to six weeks in the bursa. Recently, the presence of infectious IBDV in broilers brought to processing plants was reported. In that study bursa samples from 26 processing plants in the Eastern U.S. were examined. The IBDV specific RT-PCR detected 25.5 % of the bursal samples from 11 different processing plants. Nucleotide sequence analysis conducted on 12 RT-PCR positive samples indicated that the IBDV detected was not the commercially available attenuated vaccine strains. Five RT-PCR positive samples were selected at random for testing in specific-pathogen-free chickens. All five samples were positive for infectious 6 IBDV as confirmed by macroscopic lesions and bursa/body weight ratios. The five viruses were re-isolated and identified in bursa tissue from chickens using RT-PCR and nucleotide sequencing (Jackwood and Sommer-Wagner, 2010). 1.3 TARGET ORGAN The target organ for pathogenic serotype 1 is the bursa of fabricius (BF). The BF reaches the maximum development between 3-6 weeks of age and at this time chickens are most susceptible to the disease. The IBDV infection results in high mortality during the acute stage of the disease or in B cell deficiency after recovery from infection (Becht, 1980; Kaufer and Weiss, 1980) . Chickens infected with IBDV immediately after hatching develop a chronic infection with atrophy of BF and B cell depletion (Hudson et al., 1975; Winterfield et al., 1972). Chickens infected with IBDV when older than 12 weeks do not show clinical signs (Becht, 1980). The bursectomized chickens survive the IBDV infections which is lethal for normal chicken (Kaufer and Weiss, 1980). High concentrations of antigens and high infectivity titers were found in BF of infected chickens, whereas only traces of antigen and low virus titers were detected in the thymus, spleen (Kaufer and Weiss, 1980) and peripheral blood (Burkhardt and Muller, 1987; Mundt et al., 2003). In vitro infection studies have shown that IBDV replicates in the population of proliferating B cells (Muller, 1986; Skeeles et al., 1979b) but not in very immature lymphobalsts or competent B cells (Becht, 1980). Apathogenic serotype 2 strains do not replicate in bursal lymphoid cells or in other lymphoid cells (Nieper and Muller, 1996). Treatment of chicken with cyclophosphamide 7 (CY) or surgical bursectomy at 4 weeks of age is known to prevent IBDV infection (Kaufer and Weiss, 1980). Bursectomized chickens did not show the disease and had transient lesions in the lymphatic tissues. However, these chickens had virus and produced antibodies against it. The virus concentration in the bursectomized chickens was about 1000 times lower than the non-bursectomized chickens (Kaufer and Weiss, 1980). It appeared that the availability of a large number of bursal cells is an essential factor in the development of IBD (Ismail et al., 1987). 1.4 PATHOGENESIS Pathogenesis is defined as the method used by the virus to cause injury to the host with mortality, disease or immuno-suppression as a consequence (van den Berg et al., 2000). The injuries can be evaluated at the level of whole animal, the organ and the cell. IBDV usually infects young chickens between 3-6 weeks of age and causes a clinical disease, while sub-clinically infecting older birds. The outcome of IBDV infection is dependent on the strain and amount of the infecting virus, the age and breed of the birds, route of inoculation and presence or absence of neutralizing antibodies (Muller et al., 2003). Sequential studies of tissues from orally infected chickens using immuno-fluorescence detected the viral antigen in macrophages and lymphoid cells in the cecum at 4 hr PI and in the lymphoid cells of duodenum and jejunum at 5 hr PI (Muller et al., 1979). The virus reaches the liver at 5 hrs PI and enters the bloodstream from where it is distributed to other organs; the bursal infection is followed by viremia. The virus persists in the bursa of experimentally inoculated SPF chickens up to 3 weeks of age but the presence of 8 maternal antibodies in the commercial chicken decreases the duration of its existence in bursa (Abdel-Alim and Saif, 2001a). Various studies have shown that the variant and classic viruses exhibit similar pathology but differ from each other with respect to their pathogenicity and immunogenicity (Hassan et al., 1996). Variant viruses (Var A) were reported to induce bursal atrophy with minimal or no immune response in contrast to the classic viruses (IM) which induce a severe inflammatory response (Sharma et al., 1989). However, it was noticed subsequently that variant viruses are not homogenous as a group as thought previously (Hassan et al., 1996). Host systems used to propagate the virus have a profound effect on the pathogenicity of the virus isolates. Significant differences occurred in the pathogenicity and immunogenicity of the virus propagated in BF or in the blue grates monkey-70 (BGM70) cells. However, the antigenicity of the viruses propagated in BF or the BGM-70 cells were not significantly different (Hassan et al., 1996; Hassan and Saif, 1996). Some strains of IBDV can adapt to CEF while others are refractory to grow in it. The SAL strain was adapted and passaged successfully in CEF cells while IN strain was unable to grow in CEF (Hassan et al., 1996; Hassan and Saif, 1996). The back passage of either IN or SAL in SPF chickens maintained or increased the virulence of both viruses (Hassan et al., 1996; Hassan and Saif, 1996). Wild type viruses from B lymphocytes of BF were reported to be different than those grown in chicken embryo fibroblast (CEF). Differentiating B lymphocytes in the BF provide the optimal micro-environment for 9 highly efficient virus replication; CEF and other cells seem to lack that environment (Lange et al., 1987). 1.5 IMMUNOLOGY The IBDV is ubiquitous in commercial chickens environment and chickens acquire the infection orally or by inhalation. The virus is transferred from the gut to other tissues by phagocytic cells like macrophages. In macrophages of the gut associated tissues it could be detected as early as 4 hours after oral inoculation using immunofluorescence (Muller et al., 1979). The virus then reaches the bursa via the blood where the most extensive virus replication occurs. By 13 hours post-inoculation (PI) most follicles are positive for virus and by 16 hours PI a second and pronounced viremia occurs accompanied by secondary replication in other organs resulting in disease and death (van den Berg et al., 2000). The target organs for the virus are the IgM+ bearing B cells. During the acute phase of the disease the bursa undergoes atrophy as the bursal follicles get depleted of B cells. Virus replication causes extensive damage to lymphoid cells in medullary and cortical regions of the follicle. Apoptosis of the neighboring B cells augments the destruction of the bursal morphology. By this time an ample amount of viral antigen can be detected in other organs (Granzow et al., 1997; Kim et al., 1999). Maternally derived antibodies (MDA) protect chickens against subclinical disease and immunosuppression (Giambrone and Clay, 1986). The MDA is known to protect the chickens for 3 weeks of age (Lasher and Davis, 1997). 10 T cells are resistant to infection by IBDV(Hirai et al., 1979). During the acute phase of the disease lesions appear in the thymus which are quickly overcome within a few days (Sharma et al., 2000). A profound influx of T cells is reported in and around the site of virus replication. The infiltrated T cells could be detected from one to twelfth weeks postinoculation, although the viral antigen disappears by the third weeks. The IBDV induced cytotoxic T cell limit the spread of the virus by destroying the cells expressing the viral antigen and thus can initiate the recovery process. At the same time IBDV-induced T cells might enhance the viral lesions by producing inflammatory cytokines. T helper cells produce inflammatory cytokines like IFN-γ which activates the macrophages to produce nitric oxide (NO) (Sharma et al., 2000). Both humoral and cellular arms of the immune system are compromised during the IBDV infection due to lysis of the B cells and altered antigen-presenting cells. The IBDV causes a transient inhibition of in vitro proliferative activity of T cells to mitogens. The virus stimulates the macrophages to produce T cell cytokine like IFN-γ to produce nitric oxide (NO) and other cytokines with anti-proliferative activity. IBD did not affect natural killer cells levels in chickens (Sharma et al., 2000).The NO production after IBD virus infection exerts antiviral effect since the immune-suppressed chickens that failed to induce NO had more severe disease and higher degree of virus replication. But it does not seem to correlate with the hemorrhagic lesions which result from the reaction of host-factors and the determinants responsible for virus virulence and virus clearance (Poonia and Charan, 2005). 11 The IBDV induced damage to humoral immunity is reversible. Antibody production correlates with the morphologic restoration of the bursal follicles. Mitogenic response of T cells returned to the normal levels. During the course of mitogenic inhibition, T cells of infected chicken also failed to secrete IL-2 upon in vitro stimulation (Sharma and Fredericksen, 1987). The T cells play a significant role in the pathogenesis of IBDV. Intra bursal T cells and T-cell-mediated responses play significant role in viral clearance and promoting recovery from infection. They defend the host cell by reducing the viral burden but at the same time produce inflammatory cytokines and nitric oxide inducing factor that enhance tissues destruction and also delay the recovery process (Rautenschlein et al., 2002b). Intrabursal T cells were activated by in vitro stimulation with IBDV. The activated cells had increased surface expression of chicken MHC class II molecule, Ia and IL-2 receptor CD25. In addition, these cells have an up regulated IFN-γ gene expression (Kim et al., 2000). Splenocytes exposed to IBDV produced nitric oxide inducing factor (IFN-γ) (Rautenschlein et al., 2002b). Intrabursal T cells inhibited the mitogenic response of normal splenocytes by 90%. This bursal T cell-induced mitogen inhibition was found to be dose-dependent and not MHC-restricted (Kim and Sharma, 2000). In contrast to the bursal T cells, the splenocytes from IBDV exposed chickens did not have suppressive activity. Mitogenic inhibition by bursal T cells is mediated by soluble factors, the nature of which is still unknown (Rautenschlein et al., 2002b). Chickens that survive the disease, clear the virus and recover from its pathologic effects (Sharma et al., 2000). It has been shown that the more virulent the virus the stronger is the suppression of the humoral and 12 cell mediated immunity. Virulent virus also produced a detectable NO production in serum. Humoral immunity is the primary mechanism of the protective immune response. Infection with IBDV results in the formation of antibodies to the group and serotype specific antigens (Jackwood et al., 1985). Field exposure or vaccination results in VN titers higher than 1:1000. But weak responses are obtained in chickens immunized with purified viral polypeptides (Fahey et al., 1985), since viral protein conformation is important in eliciting a high VN antibody response (Azad et al., 1987). Antibody production is stimulated at the primary site of viral replication in gut associated tissue and they can be detected as soon as 3 days PI. These antibodies prevent the spread of the virus to other tissues. Due to the rapid onset of antibodies, the necrotic foci that form in the bursa of fabricius stop expanding and are completely eliminated (Becht, 1980). 1.6 ROLE OF T CELLS IN THE IMMUNOPATHOGENESIS OF IBDV Appearance of viral antigen in bursa is accompanied by an infiltration of T cells while IgM+ cells undergo a precipitous decrease and the immunoglobulin level remains the same (158). Infiltrating T cells were first detected at one day post-inoculation through flowcytometry and were shown to persist up to 12 weeks (Sharma et al., 2000). The ratio of CD4 and CD8 cells were the same during the seven days PI, but CD8 cells became predominant afterwards (Kim et al., 2000). The IBDV induced bursal T cells have increased surface expression of MHC-II and IL-2 receptors, with elevated expression of cytokine genes like IFN-γ and IL-6 (Sharma et al., 13 2000). T cells from the bursa of the recovered bird proliferate when exposed in vitro to purified IBDV. While the spleen cells from IBDV exposed-chicken produced nitric oxide stimulating factor when stimulated in vitro with purified IBDV. Bursal T cells also suppressed the mitogenic proliferation of the spleen from normal, virus free chicken (Rautenschlein et al., 2002b). T cell immunodeficiency can modulate pathogenicity of the virus since it has been shown that the thymectomized birds have a higher viral burden in the bursa, with lower inflammatory lesions in bursa. In addition, downregulated IFN-γ and IL-2 genes in bursal cells, have a lower incidence of apoptotic bursal cells (Sharma et al., 2000) and undergo a quick follicular recovery than T cell intact birds (Rautenschlein et al., 2002b). 1.7 EFFECT OF IBDV ON INNATE IMMUNITY IBDV have been shown to modulate the macrophage function by altering the in vitro phagocytic activity (162). Macrophages from the infected chicken have upregulated cytokine gene expression and produce increased levels of NO (Kim et al., 1998). There are reports that macrophages and monocytes may be susceptible to infection with the virus (Burkhardt and Muller, 1987; Inoue et al., 1997; Kaufer and Weiss, 1976; Kaufer and Weiss, 1980; Khatri et al., 2005; Komine et al., 1989; Palmquist et al., 2006). 1.8 IMMUNOSUPPRESSION Immunosuppression caused by IBDV has a significant economic impact due to widespread nature of the disease in commercial chickens. IBDV infection at an early age compromises the humoral and local immune responses of chickens. Allan made the earliest observation about the immunosuppressive potential of IBDV (Allan et al., 1972). 14 The extent of the immunosuppressive effect is related to the age at infection. The most pronounced damage results if the infection occurs within the first 2-3 weeks of hatch (Allan et al., 1972). Chickens less than three weeks of age do not exhibit clinical signs but are immunosuppressed (Saif, 1991). Following the ingestion of the virus, lymphoid cells and macrophages in the intestine are infected and carry the virus to the bursa of fabricius (BF) (Muller et al., 1979). Clinical signs and lesions of IBDV appear shortly thereafter. Chickens infected with IBDV are more susceptible to various other infections. Chickens exposed to IBDV at 1 day of age had lower antibody responses and were more prone to infection with Newcastle disease virus (NDV) (Faragher et al., 1972). The infected chicken had a decreased humoral antibody response to vaccines as well (Hirai et al., 1974). Immunosuppression resulted in lower flock performance, more secondary infections, poor feed conversion, less protective response to vaccines and higher rate of carcass condemnation at the processing level (Sharma et al., 2000). The immunosuppressive effects of IBDV are dependent on the strain of the virus (Craft et al., 1990; Higashihara et al., 1991; Mazariegos et al., 1990; Sharma et al., 1989). Chicken infected with IBDV at an earlier age succumbed to other infections like inclusion body hepatitis (Bacon et al., 1986), reovirus (Montgomery and Maslin, 1991), coccidiosis (Anderson et al., 1977), Marek’s disease (Sharma, 1984), hemorrhagicaplastic anemia and gangrenous dermatitis (Rosenberger et al., 1975), infectious laryngotracheitis (Rosenberger and Gelb, 1978), infectious bronchitis (Pejkovski et al., 1979), chicken anemia agent, salmonellosis, Escherichia coli, colibacillosis Mycoplasma synoviae (Giambrone et al., 1977b) and Eimeria tenella (Anderson et al., 1977; 15 Giambrone et al., 1977a). Peripheral blood lymphocytes (PBL) from chickens infected with IBDV have depressed proliferative responses to stimulation with the mitogens concanavalin A or phorbol myristate acetate (Rautenschlein et al., 2002b; Sharma et al., 2000) . 1.9 EFFECTS OF VIRUS ON HUMORAL IMMUNITY The IBDV has a predilection for the immature (Sivanandan and Maheswaran, 1980a) actively dividing B lymphocytes and causes lytic infection of IgM bearing B cells + resulting in the decrease in circulating IgM cells. Infected chicken produce less level of antibodies against the antigen (Kim et al., 1999). Only primary antibody responses are affected and secondary responses remain unaltered (Rodenberg et al., 1994; Sharma et al., 1989). IBDV induced humoral deficiency is reversible and overlaps with the restoration of bursal morphology (Sharma et al., 1989; Sharma et al., 2000). Chickens infected with IBDV at 1 day of age were found to be completely deficient in serum immunoglobulin G and produced only a monomeric immunoglobulin M (IgM) (Ivanyi, 1975; Ivanyi and Morris, 1976). The IgG levels varied depending on the age at the time of infection (Hirai et al., 1979). The number of B cells in peripheral blood was reduced after infection with IBDV, but T cells were not appreciably affected (Hirai et al., 1979; Sivanandan and Maheswaran, 1980b). The adverse effect on antibody responses is due to the damage to B cells in the bursa and in blood. Since the virus has a predilection for actively dividing B cells as compared to the mature B cells (Sivanandan and Maheswaran, 1980a). 16 1.10 EFFECT OF VIRUS ON CELLULAR IMMUNITY The extent of which cellular immune response is affected is not well understood. However, it is known that the effect of IBDV on CMI is transient and less pronounced than the effect on humoral response. Infected chickens show a poor cellular response to certain antigens and show increased susceptibility of disease that are under the control of cellular immune defense (Anderson et al., 1977). The thymic lesions were transient and appeared within the first weeks of infection, peaked at 3-4 days PI and then subsided. The presence of thymic lesions were not associated with active viral replication of the virus in the thymic cells as shown by the immunoflourescence (IF) and antigen capture ELISA. In addition, T cells from infected chickens during the early stages of virus infection fail to respond optimally to mitogens in vitro (Confer et al., 1981). Maximum depression in the cellular immunity was shown to occur at 6 weeks postinfection by using the lymphoblast transformation assay. The reason for the delay in this response is not clear considering that the virus persists in the host for approximately 3 weeks. It was speculated that this depression is the overall depression of T-cell function during the virus infection (Sivanandan and Maheswaran, 1981). The effect of IBDV on two CMI functions i.e. natural killer cell cytotoxicity and mitogenic response had been studied. It was reported that IBDV had an inconsistent effect on the natural killer cell cytotoxicity but caused a transient early depression of the blastogenic response of spleen cells to phytohemaglutinin (Sharma and Lee, 1983). In vitro mitogen hyporesponsiveness of T cells is mediated by the suppressor cells in the spleens of the infected 17 chicken; the mechanisms of reduced in vivo cellular immunocompetence are not known (Lam, 1991). The IBDV infected chickens were shown to have a normal natural killer cell population, mononuclear phagocytic activity and delayed-type hyper sensitivity reaction (Granzow et al., 1997; Hudson et al., 1975). Neither did virus infection alter the normal proportions of CD4 and CD8 subsets of T cells in the circulation and spleen (Sharma et al., 1993). It was reported that variant A strain of IBD had a significantly higher effect on CMI as compared to the standard Edgar strain when given to 1-day-old chicken which lingered on up to 5 weeks. A similar effect was reported in chicken infected at 3 weeks of age (Craft et al., 1990). Harderian gland an, another component of immune system associated with the local immune system of the respiratory tract. IBDV infection of 1-5 day-old chickens produced a dramatic decrease in plasma cell content of the harderian gland that lasted up to 7 weeks (Dohms et al., 1981). Broilers infected with IBDV at 3 weeks of age had reduced antibody titers to Brucella abortus (T cell independent antigen) and sheep red blood cells (SRBC, a T cell dependent antigen) in extracts from harderian gland and serum. Decreasing antibody responses to B. abortus were evident at a later time as compared to SRBC antibody response (Dohms et al., 1981). 1.11 MECHANISMS OF IMMUNOSUPPRESSION Reduction in the number of B cells in the BF due to viral infection is the major cause of immunosuppression. Suppression of B cell function might be caused by damage to helper T cells or other cells involved in generating the immune responses (Sharma et al., 18 1989). Chickens infected with IBDV have suppressor cells in the spleen, which cause in vitro mitogenic hypo responsiveness to concavalin A. These cells prevent normal spleen cells from responding to the mitogen (Sharma and Fredericksen, 1987). The impairment of T cells and development of suppressor cells (Sharma and Fredericksen, 1987) was demonstrated in vitro by using proliferation tests (Confer and MacWilliams, 1982; Confer et al., 1981; Sharma and Lee, 1983). 1.12 APOPTOSIS Apoptosis or the programmed cell death plays a major role in the immuno-pathology of IBDV characterized by destruction of cells of BF (Becht and Muller, 1991; Burkhardt and Muller, 1987; Kaufer and Weiss, 1980). Only 20% of the lymphoid cells in the BF contain replicating IBDV. The severe damage to the bursa can be ascribed to apoptosis (Burkhardt and Muller, 1987). In addition to necrosis, marked atrophy of the BF occurs without eliciting an inflammatory response that is a characteristic sign of the apoptotic process. Replication of the virus in BF results in secondary viremia thus spreading the virus to other tissues. It has been suggested that early post-infection, the cells containing the viral antigen are protected from apoptosis to ensure viral replication. Anti- viral mechanisms kick in and destroy the neighboring cells to prevent the spread of the virus. During the late infection, the infected cells undergo apoptosis thus seeding the virus to other cells. The IBDV infection of a susceptible chicken has been shown to induce apoptosis in the bursa as well as thymus (Inoue et al., 1994; Lam, 1997; Ojeda et al., 1997; Tanimura and Sharma, 1997; Tanimura and Sharma, 1998; Vasconcelos and Lam, 1995). 19 Morphological and biochemical features of apoptosis were also observed after in vitro infection of IBDV in chicken peripheral blood lymphocytes (Vasconcelos and Lam, 1994) and chicken embryo fibroblasts (Tham and Moon, 1996). Apoptosis occurs in lymphocytes of various organs like thymus (Inoue et al., 1994), bursa and spleen (Lam, 1997). Some researchers believed that apoptosis induced by IBDV in cell cultures following in vitro infection was an early genetic response of the host cells and was independent of virus replication while others showed that appearance of CPE coincided with virus replication (Jungmann et al., 2001; Tham and Moon, 1996). (Jungmann et al., 2001), however, showed that the proportion of apoptotic cells increased from 5.8 % at 4 hrs PI to 64.5% at 48 hrs PI in chicken embryo cells after infection with IBDV strain Cu1. However, treatment of CE cell cultures with UV inactivated IBDV did not induce apoptosis (Jungmann et al., 2001). Whether apoptosis is triggered via virus receptor activation is not yet known. Double labeling technique revealed that during the course of early infection maximum number of antigen- expressing cells were not apoptotic. It was only later in the infection that the double-labeled cells appeared. Double labeling technique determined the distribution of both apoptotic cells and cells containing viral antigen in the same section of BF. Double labeling studies for apoptotic or antigen positive cells revealed that apoptosis in bursa occurs both in IBDV positive and IBDV negative cells (Tanimura and Sharma, 1998) whereas apoptosis in the thymus occurs in the antigen negative cells only (Tanimura and Sharma, 1998). It was concluded that IBDV induced apoptosis indirectly in non-bursal organs. It has been postulated that IBDV impairs the withdrawal of 20 apoptotic cells and therefore results in the increased number of the apoptotic cells (Ojeda et al., 1997). Apoptotic cells were located mostly in an area between the cortex and medulla whereas majority of cells positive for viral antigens were found in medulla. Indirect mechanisms might also be involved in the induction of apoptosis and could have induced apoptosis in vivo resulting in rapid depletion of cells in BF. In the infected follicles large numbers of cells were apoptotic but very few contained the viral antigen (Jungmann et al., 2001; Tanimura and Sharma, 1998). Interferon production occurs after IBDV infection and is thought to be the major apoptosis-inducing factor in the neighboring cells along with TNF-α (Jungmann et al., 2001). Viral proteins VP2 and VP5 have been implicated to play a role in apoptosis. The VP2 induced apoptosis in mammalian cells but not in CE cells (Fernandez-Arias et al., 1997). The VP5 deletion mutant of IBDV induced lesser degree of apoptosis in infected CE cells and replicated slower than the parental strain (Yao et al., 1998). 1.13 REFERENCES Abdel-Alim, G.A. and Saif, Y.M. (2001) Detection and persistence of infectious bursal disease virus in specific-pathogen-free and commercial broiler chickens. Avian Dis 45(3), 646-54. Al-Natour, M.Q., Ward, L.A., Saif, Y.M., Stewart-Brown, B. and Keck, L.D. (2004) Effect of diffferent levels of maternally derived antibodies on protection against infectious bursal disease virus. Avian Dis 48(1), 177-82. Allan, W.H., Faragher, J.T. and Cullen, G.A. (1972) Immunosuppression by the infectious bursal agent in chickens immunised against Newcastle disease. Vet Rec 90(18), 511-2. 21 Anderson, W.I., Reid, W.M., Lukert, P.D. and Fletcher, O.J., Jr. (1977) Influence of infectious bursal disease on the development of immunity to Eimeria tenella. Avian Dis 21(4), 637-41. Anon. (1999) International Animal Health Code: mammals, birds and bees. Office International des Epizooties; 8th ed. Office International des Epizooties 8th ed. Ashraf, S., Abdel-Alim, G. and Saif, Y.M. (2006) Detection of antibodies against serotypes 1 and 2 infectious bursal disease virus by commercial ELISA kits. Avian Dis 50(1), 104-9. Australia. (2001. ) Generic import risk analysis (IRA) for uncooked chicken meat. IRA Report, 35. Azad, A.A., Jagadish, M.N., Brown, M.A. and Hudson, P.J. (1987) Deletion mapping and expression in Escherichia coli of the large genomic segment of a birnavirus. Virology 161(1), 145-52. Bacon, L.D., Fadly, A.M. and Crittenden, L.B. (1986) Absence of influence on immune competence by the sex-linked gene (K) determining slow feathering in white leghorn chickens. Avian Dis 30(4), 751-60. Becht, H. (1980) Infectious bursal disease virus. Curr Top Microbiol Immunol 90, 10721. Becht, H. and Muller, H. (1991) Infectious bursal disease--B cell dependent immunodeficiency syndrome in chickens. Behring Inst Mitt(89), 217-25. Burkhardt, E. and Muller, H. (1987) Susceptibility of chicken blood lymphoblasts and monocytes to infectious bursal disease virus (IBDV). Arch Virol 94(3-4), 297303. Chettle, N., Stuart, J.C. and Wyeth, P.J. (1989) Outbreak of virulent infectious bursal disease in East Anglia. Vet Rec 125(10), 271-2. Confer, A.W. and MacWilliams, P.S. (1982) Correlation of hematological changes and serum and monocyte inhibition with the early suppression of phytohemagglutinin stimulation of lymphocytes in experimental infectious bursal disease. Can J Comp Med 46(2), 169-75. Confer, A.W., Springer, W.T., Shane, S.M. and Donovan, J.F. (1981) Sequential mitogen stimulation of peripheral blood lymphocytes from chickens inoculated with infectious bursal disease virus. Am J Vet Res 42(12), 2109-13. 22 Craft, D.W., Brown, J. and Lukert, P.D. (1990) Effects of standard and variant strains of infectious bursal disease virus on infections of chickens. Am J Vet Res 51(8), 1192-7. Delgui, L., Gonzalez, D. and Rodriguez, J.F. (2009) Infectious bursal disease virus persistently infects bursal B-lymphoid DT40 cells. J Gen Virol 90(Pt 5), 1148-52. Dohms, J.E. and Jaeger, J.S. (1988) The effect of infectious bursal disease virus infection on local and systemic antibody responses following infection of 3-weeks-old broiler chickens. Avian Dis 32(4), 632-40. Dohms, J.E., Lee, K.P. and Rosenberger, J.K. (1981) Plasma cell changes in the gland of Harder following infectious bursal disease virus infection of the chicken. Avian Dis 25(3), 683-95. Elankumaran, S., Heckert, R.A. and Moura, L. (2002) Pathogenesis and tissue distribution of a variant strain of infectious bursal disease virus in commercial broiler chickens. Avian Dis 46(1), 169-76. Fahey, K.J., O'Donnell, I.J. and Bagust, T.J. (1985) Antibody to the 32K structural protein of infectious bursal disease virus neutralizes viral infectivity in vitro and confers protection on young chickens. J Gen Virol 66 ( Pt 12), 2693-702. Faragher, J.T., Allan, W.H. and Cullen, G.A. (1972) Immunosuppressive effect of the infectious bursal agent in the chicken. Nat New Biol 237(73), 118-9. Fernandez-Arias, A., Martinez, S. and Rodriguez, J.F. (1997) The major antigenic protein of infectious bursal disease virus, VP2, is an apoptotic inducer. J Virol 71(10), 8014-8. Flensburg, M.F., Ersboll, A.K. and Jorgensen, P.H. (2002) Risk factors associated with the introduction of acute clinical infectious bursal disease among Danish broiler chickens in 1998. Avian Pathol 31(1), 23-9. Gardner, H., Kerry, K., Riddle, M., Brouwer, S. and Gleeson, L. (1997) Poultry virus infection in Antarctic penguins. Nature 387(6630), 245. Giambrone, J.J., Anderson, W.I., Reid, W.M. and Eidson, C.S. (1977a) Effect of infectious bursal disease on the severity of Eimeria tenella infections in broiler chicks. Poult Sci 56(1), 243-6. Giambrone, J.J. and Clay, R.P. (1986) Evaluation of the immunogenicity, stability, pathogenicity, and immunodepressive potential of four commercial live infectious bursal disease vaccines. Poult Sci 65(7), 1287-90. 23 Giambrone, J.J., Eidson, C.S. and Kleven, S.H. (1977b) Effect of infectious bursal disease on the response of chickens to Mycoplasma synoviae, Newcastle disease virus, and infectious bronchitis virus. Am J Vet Res 38(2), 251-3. Granzow, H., Birghan, C., Mettenleiter, T.C., Beyer, J., Kollner, B. and Mundt, E. (1997) A second form of infectious bursal disease virus-associated tubule contains VP4. J Virol 71(11), 8879-85. Hassan, M.K., Al-Natour, M.Q., Ward, L.A. and Saif, Y.M. (1996) Pathogenicity, attenuation, and immunogenicity of infectious bursal disease virus. Avian Dis 40(3), 567-71. Hassan, M.K. and Saif, Y.M. (1996) Influence of the host system on the pathogenicity, immunogenicity, and antigenicity of infectious bursal disease virus. Avian Dis 40(3), 553-61. Henderson, K.S. and Jackwood, D.J. (1990) Comparison of the dot blot hybridization assay with antigen detection assays for the diagnosis of infectious bursal disease virus infections. Avian Dis 34(3), 744-8. Higashihara, M., Saijo, K., Fujisaki, Y. and Matumoto, M. (1991) Immunosuppressive effect of infectious bursal disease virus strains of variable virulence for chickens. Vet Microbiol 26(3), 241-8. Hirai, K., Funakoshi, T., Nakai, T. and Shimakura, S. (1981) Sequential changes in the number of surface immunoglobulin-bearing B lymphocytes in infectious bursal disease virus-infected chickens. Avian Dis 25(2), 484-96. Hirai, K., Kunihiro, K. and Shimakura, S. (1979) Characterization of Immunosuppression in chickens by infectious bursal disease virus. Avian Dis 23(4), 950-65. Hirai, K., Shimakura, S., Kawamoto, E., Taguchi, F., Kim, S.T., Chang, C.N. and Iritani, Y. (1974) The immunodepressive effect of infectious bursal disease virus in chickens. Avian Dis 18(1), 50-7. Hudson, L., Pattison, M. and Thantrey, N. (1975) Specific B lymphocyte suppression by infectious bursal agent (Gumboro disease virus) in chickens. Eur J Immunol 5(10), 675-9. Inoue, M., Fukuda, M. and Miyano, K. (1994) Thymic lesions in chicken infected with infectious bursal disease virus. Avian Dis 38(4), 839-46. Inoue, M., Satoh, W. and Murakami, H. (1997) Plasma alpha 1-acid glycoprotein in chickens infected with infectious bursal disease virus. Avian Dis 41(1), 164-70. 24 Ismail, N.M., Fadly, A.M. and Chang, T.S. (1987) Effect of bursal cell number on the pathogenesis of infectious bursal disease in chickens. Avian Dis 31(3), 546-55. Ismail, N.M., Saif, Y.M., Wigle, W.L., Havenstein, G.B. and Jackson, C. (1990) Infectious bursal disease virus variant from commercial Leghorn pullets. Avian Dis 34(1), 141-5. Ivanyi, J. (1975) Immunodeficiency in the chicken. II. Production of monomeric IgM following testosterone treatment or infection with Gumboro disease. Immunology 28(6), 1015-21. Ivanyi, J. and Morris, R. (1976) Immunodeficiency in the chicken. IV. An immunological study of infectious bursal disease. Clin Exp Immunol 23(1), 154-65. Jackwood, D.H. and Saif, Y.M. (1987) Antigenic diversity of infectious bursal disease viruses. Avian Dis 31(4), 766-70. Jackwood, D.J., Saif, Y.M. and Moorhead, P.D. (1985) Immunogenicity and antigenicity of infectious bursal disease virus serotypes I and II in chickens. Avian Dis 29(4), 1184-94. Jackwood, D.J. and Sommer-Wagner, S.E. (2005) Molecular epidemiology of infectious bursal disease viruses: distribution and genetic analysis of newly emerging viruses in the United States. Avian Dis 49(2), 220-6. Jackwood, D.J. and Sommer-Wagner, S.E. (2010) Detection and characterization of infectious bursal disease viruses in broilers at processing. Prev Vet Med 97(1), 45-50. Jackwood, D.J., Sommer-Wagner, S.E., Stoute, A.S., Woolcock, P.R., Crossley, B.M., Hietala, S.K. and Charlton, B.R. (2009) Characteristics of a very virulent infectious bursal disease virus from California. Avian Dis 53(4), 592-600. Jungmann, A., Nieper, H. and Muller, H. (2001) Apoptosis is induced by infectious bursal disease virus replication in productively infected cells as well as in antigennegative cells in their vicinity. J Gen Virol 82(Pt 5), 1107-15. Kaufer, I. and Weiss, E. (1976) Electron-microscope studies on the pathogenesis of infectious bursal disease after intrabursal application of the causal virus. Avian Dis 20(3), 483-95. Kaufer, I. and Weiss, E. (1980) Significance of bursa of Fabricius as target organ in infectious bursal disease of chickens. Infect Immun 27(2), 364-7. 25 Kawai, T. and Akira, S. (2006) Innate immune recognition of viral infection. Nat Immunol 7(2), 131-7. Kawai, T. and Akira, S. (2009) The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol 21(4), 317-37. Khatri, M., Palmquist, J.M., Cha, R.M. and Sharma, J.M. (2005) Infection and activation of bursal macrophages by virulent infectious bursal disease virus. Virus Res 113(1), 44-50. Kim, I.J., Gagic, M. and Sharma, J.M. (1999) Recovery of antibody-producing ability and lymphocyte repopulation of bursal follicles in chickens exposed to infectious bursal disease virus. Avian Dis 43(3), 401-13. Kim, I.J., Karaca, K., Pertile, T.L., Erickson, S.A. and Sharma, J.M. (1998) Enhanced expression of cytokine genes in spleen macrophages during acute infection with infectious bursal disease virus in chickens. Vet Immunol Immunopathol 61(2-4), 331-41. Kim, I.J. and Sharma, J.M. (2000) IBDV-induced bursal T lymphocytes inhibit mitogenic response of normal splenocytes. Vet Immunol Immunopathol 74(1-2), 47-57. Kim, I.J., You, S.K., Kim, H., Yeh, H.Y. and Sharma, J.M. (2000) Characteristics of bursal T lymphocytes induced by infectious bursal disease virus. J Virol 74(19), 8884-92. Komine, K., Ohta, H., Kamata, S., Uchida, K., Yoshikawa, Y., Yamanouchi, K. and Okada, H. (1989) Complement activation by infectious bursal disease virus and its relevance to virus growth in the lymphoid cells. Nippon Juigaku Zasshi 51(6), 1259-62. Lam, K.M. (1991) Infectious bursal disease virus type 1-induced suppression of chicken lymphocyte response to mitogen. Avian Pathol 20(2), 205-12. Lam, K.M. (1997) Morphological evidence of apoptosis in chickens infected with infectious bursal disease virus. J Comp Pathol 116(4), 367-77. Lange, H., Muller, H., Kaufer, I. and Becht, H. (1987) Pathogenic and structural properties of wild type infectious bursal disease virus (IBDV) and virus grown in vitro. Arch Virol 92(3-4), 187-96. Lasher, H.N. and Davis, V.S. (1997) History of infectious bursal disease in the U.S.A.-the first two decades. Avian Dis 41(1), 11-9. 26 Lim, B.L., Cao, Y., Yu, T. and Mo, C.W. (1999) Adaptation of very virulent infectious bursal disease virus to chicken embryonic fibroblasts by site-directed mutagenesis of residues 279 and 284 of viral coat protein VP2. J Virol 73(4), 2854-62. Luque, D., Rivas, G., Alfonso, C., Carrascosa, J.L., Rodriguez, J.F. and Caston, J.R. (2009) Infectious bursal disease virus is an icosahedral polyploid dsRNA virus. Proc Natl Acad Sci U S A 106(7), 2148-52. Mackenzie, M. and Spradbrow, P.B. (1981) Persistence of infectious bursal disease virus in experimentally infected chickens. Aust Vet J 57(11), 534-5. Mandeville, W.F., Cook, F.K. and Jackwood, D.J. (2000) Heat lability of five strains of infectious bursal disease virus. Poult Sci 79(6), 838-42. Mazariegos, L.A., Lukert, P.D. and Brown, J. (1990) Pathogenicity and immunosuppressive properties of infectious bursal disease "intermediate" strains. Avian Dis 34(1), 203-8. McFerran, J.B., M. S. Mcnulty, F. R. Mckillop, T. J. Connor, R. M. Mccracken, D. S. Collins, and G. M. Allan. . (1980) Isolation and serological studies with infectious bursal disease viruses from fowl, turkeys and ducks— demonstration of a 2nd serotype. . Avian Pathol 9, 395–404. Montgomery, R.D. and Maslin, W.R. (1991) Effect of infectious bursal disease virus vaccines on persistence and pathogenicity of modified live reovirus vaccines in chickens. Avian Dis 35(1), 147-57. Muller, H. (1986) Replication of infectious bursal disease virus in lymphoid cells. Arch Virol 87(3-4), 191-203. Muller, H., Islam, M.R. and Raue, R. (2003) Research on infectious bursal disease--the past, the present and the future. Vet Microbiol 97(1-2), 153-65. Muller, R., Kaufer, I., Reinacher, M. and Weiss, E. (1979) Immunofluorescent studies of early virus propagation after oral infection with infectious bursal disease virus (IBDV). Zentralbl Veterinarmed B 26(5), 345-52. Mundt, E., de Haas, N. and van Loon, A.A. (2003) Development of a vaccine for immunization against classical as well as variant strains of infectious bursal disease virus using reverse genetics. Vaccine 21(31), 4616-24. Nieper, H. and Muller, H. (1996) Susceptibility of chicken lymphoid cells to infectious bursal disease virus does not correlate with the presence of specific binding sites. J Gen Virol 77 ( Pt 6), 1229-37. 27 Ojeda, F., Skardova, I., Guarda, M.I., Ulloa, J. and Folch, H. (1997) Proliferation and apoptosis in infection with infectious bursal disease virus: a flow cytometric study. Avian Dis 41(2), 312-6. Palmquist, J.M., Khatri, M., Cha, R.M., Goddeeris, B.M., Walcheck, B. and Sharma, J.M. (2006) In vivo activation of chicken macrophages by infectious bursal disease virus. Viral Immunol 19(2), 305-15. Pejkovski, C., Davelaar, F.G. and Kouwenhoven, B. (1979) Immunosuppressive effect of infectious bursal disease virus on vaccination against infectious bronchitis. Avian Pathol 8(1), 95-106. Peters, M.A., Lin, T.L. and Wu, C.C. (2005) Real-time RT-PCR differentiation and quantitation of infectious bursal disease virus strains using dual-labeled fluorescent probes. J Virol Methods 127(1), 87-95. Poonia, B. and Charan, S. (2005) Early and transient induction of nitric oxide (NO) in infectious bursal disease virus infection is T-cell dependent: a study in cyclosporin-A treated chicken-model. Indian J Exp Biol 43(2), 192-6. Rauf, A., Khatri, M., Murgia, M.V. and Saif, Y.M. (2011) Expression of perforingranzyme pathway genes in the bursa of infectious bursal disease virus-infected chickens. Dev Comp Immunol. Rautenschlein, S., Yeh, H.Y. and Sharma, J.M. (2002) The role of T cells in protection by an inactivated infectious bursal disease virus vaccine. Vet Immunol Immunopathol 89(3-4), 159-67. Reed, L.J. and Muench, H.A. (1983) A simple method of estimation fifty per cent endpoint. . Am. J. Hyg 27, 493-497. Rodenberg, J., Sharma, J.M., Belzer, S.W., Nordgren, R.M. and Naqi, S. (1994) Flow cytometric analysis of B cell and T cell subpopulations in specific-pathogen-free chickens infected with infectious bursal disease virus. Avian Dis 38(1), 16-21. Rosenberger, J.K. and Gelb, J., Jr. (1978) Response to several avian respiratory viruses as affected by infectious bursal disease virus. Avian Dis 22(1), 95-105. Rosenberger, J.K., Klopp, S., Eckroade, R.J. and Krauss, W.C. (1975) The roles of the infectious bursal agent and several avian adenoviruses in the hemorrhagicaplastic-anemia syndrome and gangrenous dermatitis. Avian Dis 19(4), 717-29. Saif, Y.M. (1991) Immunosuppression induced by infectious bursal disease virus. Vet Immunol Immunopathol 30(1), 45-50. 28 Saif, Y.M. (1998) Infectious bursal disease and hemorrhagic enteritis. Poult Sci 77(8), 1186-9. Saif, Y.M. (2004) Control of infectious bursal disease virus by vaccination. Dev Biol (Basel) 119, 143-6. Sanchez, J., Stryhn, H., Flensburg, M., Ersboll, A.K. and Dohoo, I. (2005) Temporal and spatial analysis of the 1999 outbreak of acute clinical infectious bursal disease in broiler flocks in Denmark. Prev Vet Med 71(3-4), 209-23. Sang, Y., Ross, C.R., Rowland, R.R. and Blecha, F. (2008) Toll-like receptor 3 activation decreases porcine arterivirus infection. Viral Immunol 21(3), 303-13. Sharma, J.M. (1984) Effect of infectious bursal disease virus on protection against Marek's disease by turkey herpesvirus vaccine. Avian Dis 28(3), 629-40. Sharma, J.M., Dohms, J., Walser, M. and Snyder, D.B. (1993) Presence of lesions without virus replication in the thymus of chickens exposed to infectious bursal disease virus. Avian Dis 37(3), 741-8. Sharma, J.M., Dohms, J.E. and Metz, A.L. (1989) Comparative pathogenesis of serotype 1 and variant serotype 1 isolates of infectious bursal disease virus and their effect on humoral and cellular immune competence of specific-pathogen-free chickens. Avian Dis 33(1), 112-24. Sharma, J.M. and Fredericksen, T.L. (1987) Mechanism of T cell immunosuppression by infectious bursal disease virus of chickens. Prog Clin Biol Res 238, 283-94. Sharma, J.M., Kim, I.J., Rautenschlein, S. and Yeh, H.Y. (2000) Infectious bursal disease virus of chickens: pathogenesis and immunosuppression. Dev Comp Immunol 24(2-3), 223-35. Sharma, J.M. and Lee, L.F. (1983) Effect of infectious bursal disease on natural killer cell activity and mitogenic response of chicken lymphoid cells: role of adherent cells in cellular immune suppression. Infect Immun 42(2), 747-54. Sivanandan, V. and Maheswaran, S.K. (1980a) Immune profile of infectious bursal disease (IBD). II. Effect of IBD virus on pokeweed-mitogen-stimulated peripheral blood lymphocytes of chickens. Avian Dis 24(3), 734-42. Sivanandan, V. and Maheswaran, S.K. (1980b) Immune profile of infectious bursal disease: I. Effect of infectious bursal disease virus on peripheral blood T and B lymphocytes of chickens. Avian Dis 24(3), 715-25. 29 Sivanandan, V. and Maheswaran, S.K. (1981) Immune profile of infectious bursal disease. III. Effect of infectious bursal disease virus on the lymphocyte responses to phytomitogens and on mixed lymphocyte reaction of chickens. Avian Dis 25(1), 112-20. Skeeles, J.K., Lukert, P.D., De Buysscher, E.V., Fletcher, O.J. and Brown, J. (1979a) Infectious bursal disease viral infections. I. Complement and virus-neutralizing antibody response following infection of susceptible chickens. Avian Dis 23(1), 95-106. Skeeles, J.K., Lukert, P.D., De Buysscher, E.V., Fletcher, O.J. and Brown, J. (1979b) Infectious bursal disease viral infections. II. The relationship of age, complement levels, virus-neutralizing antibody, clotting, and lesions. Avian Dis 23(1), 107-17. Stoute, S.T., Jackwood, D.J., Sommer-Wagner, S.E., Cooper, G.L., Anderson, M.L., Woolcock, P.R., Bickford, A.A., Senties-Cue, C.G. and Charlton, B.R. (2009) The diagnosis of very virulent infectious bursal disease in California pullets. Avian Dis 53(2), 321-6. Takeuchi, O. and Akira, S. (2009) Innate immunity to virus infection. Immunol Rev 227(1), 75-86. Tanimura, N. and Sharma, J.M. (1997) Appearance of T cells in the bursa of Fabricius and cecal tonsils during the acute phase of infectious bursal disease virus infection in chickens. Avian Dis 41(3), 638-45. Tanimura, N. and Sharma, J.M. (1998) In-situ apoptosis in chickens infected with infectious bursal disease virus. J Comp Pathol 118(1), 15-27. Tham, K.M. and Moon, C.D. (1996) Apoptosis in cell cultures induced by infectious bursal disease virus following in vitro infection. Avian Dis 40(1), 109-13. Vakharia, V.N., Snyder, D.B., Lutticken, D., Mengel-Whereat, S.A., Savage, P.K., Edwards, G.H. and Goodwin, M.A. (1994) Active and passive protection against variant and classic infectious bursal disease virus strains induced by baculovirusexpressed structural proteins. Vaccine 12(5), 452-6. van den Berg, T.P., Eterradossi, N., Toquin, D. and Meulemans, G. (2000) Infectious bursal disease (Gumboro disease). Rev Sci Tech 19(2), 509-43. Vasconcelos, A.C. and Lam, K.M. (1994) Apoptosis induced by infectious bursal disease virus. J Gen Virol 75 ( Pt 7), 1803-6. 30 Vasconcelos, A.C. and Lam, K.M. (1995) Apoptosis in chicken embryos induced by the infectious bursal disease virus. J Comp Pathol 112(4), 327-38. Werts, C., Girardin, S.E. and Philpott, D.J. (2006) TIR, CARD and PYRIN: three domains for an antimicrobial triad. Cell Death Differ 13(5), 798-815. Winterfield, R.W., Fadly, A.M. and Bickford, A. (1972) Infectivity and distribution of infectious bursal disease virus in the chicken. Persistence of the virus and lesions. Avian Dis 16(3), 622-32. Withers, D.R., Young, J.R. and Davison, T.F. (2005) Infectious bursal disease virusinduced immunosuppression in the chick is associated with the presence of undifferentiated follicles in the recovering bursa. Viral Immunol 18(1), 127-37. Wyeth, P.J. and Cullen, G.A. (1978) Susceptibility of chicks to infectious bursal disease (IBD) following vaccination of their parents with live IBD vaccine. Vet Rec 103(13), 281-2. Wyeth, P.J., O'Brien, J.D. and Cullen, G.A. (1981) Improved performance of progeny of broiler parent chickens vaccinated with infectious bursal disease oil-emulsion vaccine. Avian Dis 25(1), 228-41. Yao, K., Goodwin, M.A. and Vakharia, V.N. (1998) Generation of a mutant infectious bursal disease virus that does not cause bursal lesions. J Virol 72(4), 2647-54. Yokota, S., Okabayashi, T. and Fujii, N. (2010) The battle between virus and host: modulation of Toll-like receptor signaling pathways by virus infection. Mediators Inflamm 2010, 184328. 31 CHAPTER 2 PERSISTENCE AND TISSUE DISTRIBUTION OF INFECTIOUS BURSAL DISEASE VIRUS IN SPF AND COMMERCIAL BROILER CHICKENS 2.1 SUMMARY Infectious bursal disease virus (IBDV) is an important poultry pathogen that primarily infects young chickens and leads to severe immunosuppression. There have been questions regarding the persistence of IBDV in processed meat destined for export. This study was initiated to determine the persistence, distribution and quantification of IBDV strains in lymphoid and non-lymphoid tissues of specific pathogen free chickens (SPF) and commercial broiler chickens. Five separate experiments were conducted using 2 and 4 weeks old SPF chickens and 2 weeks old commercial broilers having maternally derived antibodies. Four different types of lymphoid and seven different non-lymphoid tissues were sampled from individual chickens in each experiment. Viral RNA (vRNA) 30 was extracted from tissues and subjected to real time RT-PCR. In lymphoid tissues the virus strains were detected for the longest duration in bursal tissues followed by spleen, thymus and bone marrow. In non-lymphoid tissues both of the strains were detected the longest in caecum followed by liver, kidney, pancreas, lungs, thigh muscles and breast muscles. The STC strain, a serotype 1 classic virus, vRNA was detected in bursa of SPF chickens up to 8 weeks post-inoculation (PI) and in commercial broiler chickens it was detected up to 6 weeks PI. In addition, we were able to isolate the STC strain up to 4 and 3 weeks PI from the bursal homogenates of SPF and commercial chickens respectively. The IN strain, vRNA was detected up to 5 weeks PI in SPF and up to 3 weeks PI from commercial chickens in bursal tissue. The IN strain was isolated up to 10 days postinoculation (DPI) from bursal tissues collected from both SPF and commercial chickens. This is the first detailed report on the persistence and distribution of classic and variant strains of IBDV in different tissues of SPF and commercial chickens. The study indicated that the persistence and distribution of IBDV in non-lymphoid tissues, specifically, thigh muscles and breast muscles is significantly different between SPF and commercial chickens. In commercial broilers the STC was detected at DPI 3 in thigh muscles and only one breast muscle sample was RT-PCR positive at DPI 14. IN strain was not detected in thigh muscles while it was detected in breast muscles up to two weeks PI in commercial chickens. Whereas in SPF chickens STC and IN strains, vRNA were detected in thigh muscles up to 4 and 2 weeks PI and in breast muscles up to 3 and 2 weeks PI, respectively. Although the virus can persist in SPF chicken bursa for 4 weeks, it is very 31 unlikely that the infectious virus will be present in the processed meat. In addition, the RT-PCR results are not sufficient to indicate the presence of the infectious virus. 2.2 INTRODUCTION Infectious bursal disease (IBD) is one the most important chicken diseases that affects young chickens around the world. IBD virus is an icosahedral, bisegmented and polyploid virus. Its characteristics of being polyploid and having bisegmented genome continue to present us with new challenges as it can genetically mutate to avoid vaccine immunity and can also reassort its genome to become more virulent virus (Luque et al., 2009). The IBD is considered ―communicable disease‖ and is of socio-economic importance within countries and important in the international trade of poultry products (Australia, 2001 ). Presence of IBDV in chicken meat is questioned in international trade of poultry products. IBDV being a non zoonotic pathogen is ―not a human food safety issue, nevertheless movement of birds with IBDV infections is cause for concern because the introduction of new antigenic and pathogenic strains into a geographic area can have a negative economic impact on the chickens grown in that region. Contaminating trucks, processing plant personnel and equipment servicing multiple poultry farms can lead to an increased risk of spreading IBDV‖ (Jackwood and Sommer-Wagner, 2010). Following an infection, the persistence of IBDV in chickens has been reported and the results were dependent on the type of test used. Using molecular assays, the RNA of IBDV was detected in bursa tissue at 24 days post-inoculation (Henderson and Jackwood, 1990). In a later study, viral RNA was detected by RT-PCR up to 28 DPI in bursa tissue 32 but using embryonated eggs and specific-pathogen-free chickens, infectious virus was only detected for 7–14 days post-inoculation in the bursa tissue (Abdel-Alim and Saif, 2001a). In that study, the persistence of the virus was dependent on the titer of the inoculums and quantity of maternal antibodies. In another study, it was documented that variant IBDV was detected in virus-challenged commercial broilers for up to 6 weeks and infectious virus capable of replication could be isolated from all organs at 4 weeks PI (Elankumaran et al., 2002). Control strategies for IBD include vaccination of broiler breeder flocks and the resulting transfer of maternal immunity that protect their progeny. When this strategy is successful, maternal immunity should prevent IBDV infection for several weeks following hatch (Al-Natour et al., 2004; Wyeth and Cullen, 1978). This is an effective control strategy because it protects the young chick’s immune system from being permanently compromised (Saif, 2004). In regions where IBDV is endemic, broiler chicks will become infected with the virus as maternal immunity wanes (Wyeth et al., 1981). During a study of the distribution of IBDV in the United States it was typically observed that IBDV infects the broilers at 3–6 weeks of age (Jackwood and Sommer-Wagner, 2005). Most of the studies conducted on the persistence of IBDV in tissues were on specific pathogen free (SPF) chickens. Such data does not reflect the situation in young commercial broiler chickens which usually have maternal antibodies. Hence to understand the pathogenesis and epidemiology of IBDV it was necessary to undertake this comprehensive study. The goal of this study was to determine persistence and distribution of IBDV in chicken tissues. 33 2.3 MATERIALS AND METHODS Chicken and embryonated eggs: One day old commercial broiler chicks (in ovo vaccinated used in experiment 3 and 4 and commercial broiler chicks having maternally derived antibodies used in experiment 5) were purchased from a local hatchery. The specific pathogen free (SPF) chicken eggs (Charles River Laboratories Inc; Wilmington, MA, USA) were incubated in our facility at Ohio Agriculture Research and Development Center, The Ohio State University. The hatched chickens were kept at a disease containment building that had rooms supplied with HEPA filtered intake and exhaust air. At two weeks of age (experiment 1, 3, and 5) and 4 -weeks of age (experiment 2 and 4) the birds were transferred to hard sided isolators supplied with HEPA filtered intake and exhaust air. Viruses: A serotype 1 classical IBDV (cIBDV), standard challenge (STC) strain (Jackwood and Saif, 1987) and a serotype 1 variant IBDV (vIBDV) strain IN (Ismail et al., 1990) isolated in our laboratory were used for challenge studies. Each virus strain was propagated separately in two weeks old SPF chickens. Bursas were harvested from inoculated birds at 5 DPI, homogenized aseptically, and 10 % W/V suspension was made in minimal essential medium (MEM). The bursal homogenates were titrated in 10 day old SPF chicken embryonated eggs via chorioallantioc membrane (CAM) route as described earlier (Abdel-Alim and Saif, 2001a). The titer was expressed as mean embryo infectious dose50 (EID50) per milliliter (ml) on the basis of lesions and mortality and was calculated by the method of Reed and Muench (Reed and Muench, 1983). The bursal homogenates were filtered and stored at -70 OC for later use. 34 Experimental Design: Five separate experiments were conducted. Each experiment included three groups. Group A was challenged, intraocularly with STC strain, group B was challenged intraocularly with IN strain of IBDV and groups C was kept as negative control. SPF chickens were used in experiments 1 and 2. In ovo vaccinated commercial broiler chickens were used in experiment 3 and 4. In experiment 5 commercial chickens having maternally derived antibodies were used. In experiment 1, 81 two weeks old SPF chickens were allocated equally into three groups. Group A, chickens were challenged each with 104EID50 of STC strain. Groups B, chickens were challenged each with 104EID50 of IN strain and group C 27 chickens served as virus free control. The lymphoid tissues (thymus, bursa, spleen and bone marrow) and non-lymphoid tissues (thigh muscles, breast muscles, pancreas, kidney, caecum, liver and lungs) were collected at the following time point: 3, 5, 7, 10, 14, 21, 28, 35 and 42 DPI. Three chickens per group were euthanized at each designated time points. Each tissue (approximately 2 grams in weight) was collected aseptically using separate sterile surgical scissors and forceps. The same procedure as mentioned for experiment 1 was used for experiment 2, 3, 4, and 5 with the following modifications. In the second experiment the SPF chickens were inoculated at 4 weeks of age and the samples were collected at DPI 7, 14, 21, 28, 35, 42, 49, and 56. Experiments 3 and 4 were conducted on in ovo vaccinated commercial broilers whereas in experiment 5 commercial broiler having maternally derived antibodies were used. The sampling procedure for experiment 3, 4 and 5 was similar to that of experiment 2. 35 Tissues processing: Each tissue was suspended in sterile phosphate buffered saline (PBS) in 14 ml sterile tube (Fisher scientific, USA), properly labeled for every tissue. The tissues were homogenized separately using Labgen homogenizer (Cole-Parmer, USA) supplied with sterile disposable plastic probes to rule out any sample to sample carry over cross contamination. After homogenization the tissues were frozen and thawed three times and stored at -70 O C. The homogenized tissues were subjected to centrifugation at 4000 RPM (Allegra X- 15 R centrifuge Beckman Coulter) for 20 minutes at 4 OC. The clarified supernatant from each sample was collected and used for virus isolation and viral RNA extraction. Viral RNA extraction and Real-time RT PCR: The processed tissue samples were subjected to viral RNA extraction by using the Mag MAXTM magnetic beads processor and Mag MAXTM viral RNA extraction kit as per manufacturer’s instructions (Applied Biosystems). Real- time RT-PCR was performed on all the collected tissues, using published strain specific primers and probe sets (Peters et al., 2005). Real-Time RT-PCR was performed using 7500 ABI RT-PCR system on 96 well plates. One step RT-PCR AgPath-IDTM kit (Applied Biosystems) was used for the RT-PCR reaction following the manufacturer’s instructions. The reaction profile used was: 45 oC for 10 minutes, 95 oC for 10 minutes for the RT step and 40 cycles of 95 oC for 15 seconds, 60 oC for 45 seconds for the PCR step (Rauf et al., 2011). Standard Curve generation: Serial 10 fold dilutions of RNA of known viral titer for STC and IN strains were made and standard curves were generated. Viral RNA load in 36 each tissue (approximately 2 grams in weight) was then estimated based on the standard curves developed (Rauf et al., 2011). Virus Isolation: All RT-PCR positive bursal tissues were subjected to virus isolation using ten day old SPF embryonated chicken eggs. Additionally RT-PCR positive bursal homogenates were inoculated into 2 weeks old SPF chickens to further confirm the presence of infectious virus. ELISA procedure: In each experiment blood samples were collected from chickens before inoculation and at each sampling point after inoculation with virus. Commercially available IBDV ELISA kit (IDEXX laboratories) was used. The sera were diluted, ELISA conducted and titers calculated in accordance with the manufacturer’s recommendations. Geometric mean titer (GMT) was calculated as previously published (Ashraf et al., 2006). Statistical analysis: Statistical analysis: Data was analyzed by STATA using multivariate analysis and generalized linear model. 2.4 RESULTS Persistence and tissue distribution of STC and IN strains in SPF chickens: In experiment 1, the STC vRNA was detected in bursal tissues up to 42 DPI and in spleen, thymus and bone marrow up to 35 DPI. The IN strain vRNA was detected up to 35 DPI in bursal tissues, up to 28 DPI in spleen and up to 14 DPI in thymus and bone marrow (Table; 2.1). In non-lymphoid tissues the STC vRNA was detected in thigh muscles and lungs up to 28 DPI and in breast muscles, pancreas, kidneys, caecum and 37 liver up to 35 DPI. The IN strain vRNA was detected in caecum up to 28 DPI, in thigh muscles, breast muscle, pancreas, kidneys, and liver up to 14 DPI. The IN strain vRNA was detected in lungs up to 7 DPI (Table; 2.2-3). In experiment 2, the STC strain vRNA was detected in bursas and spleens up to 56 DPI, in bone marrow up to 49 DPI and in thymus up to 35 DPI. The IN strain vRNA was detected up to 28 DPI in bursal tissues, up to 21 DPI in spleens, up to 7 DPI in bone marrow and was not detected in thymus (Table; 2.4). In non-lymphoid tissues the STC strain vRNA was detected in thigh muscles and breast muscles up to 28 DPI, in pancreas up to 35 DPI, in kidneys, caecum and liver up to 42 DPI. The IN strain vRNA was detected in caecum up to 21 DPI, in pancreas and liver up to 7 DPI. The IN strain vRNA was not detected in breast muscle, lungs and kidneys. The IN strain vRNA was detected in thigh muscles samples at DPI 28 and 35 (Table; 2.5-6). Persistence and tissue distribution of STC and IN strains in ovo vaccinated commercial broilers: In experiment 3, the STC vRNA was detected up to 35 DPI in bursa and spleen, in thymus up to 5 DPI and in bone marrow up to 28 DPI. The IN strain vRNA was detected up to 28 DPI in bursa, 21 DPI in spleen and 7 DPI in thymus, while it was not detected in bone marrow (Table; 2.7). In non-lymphoid tissues STC strain vRNA was detected in caecum and liver up to 35 DPI, in thigh muscles and lungs up to 14 DPI, pancreas and kidneys up to 10 DPI and in breast muscles was not detected. The IN strain vRNA was not detected in breast muscles pancreas and lungs at any DPI. The IN strain vRNA was 38 detected in caecum up to 21 DPI, and in thigh muscle and liver it was detectable up to 3 DPI (Table; 2.8-9). In experiment 4 the STC strain vRNA was detected up to 42 DPI in bursa and thymus, and up to 28 DPI in spleen and bone marrow (Table; 2.10). The IN strain vRNA was detected up to 42 DPI in bursa, 35 DPI in thymus and 21 DPI in spleen, while it was not detected in bone marrow. In non-lymphoid tissues STC strain vRNA was detected in thigh muscles, caecum and pancreas up to 35 DPI, in kidney, lungs and liver up to 28 DPI and in breast muscles up to DPI 7. The IN strain, vRNA, was not detected in thigh and breast muscles. The IN strain, vRNA was detected in pancreas, kidney and caecum up to DPI 28, and in liver it was detected only at DPI 3 (Table; 2.11-12). Persistence and tissue distribution of STC and IN strains in commercial broilers: In experiment 5 commercial broilers having maternally derived antibodies were inoculated with the two strains (STC and IN). The STC strain vRNA was detected up to 42 DPI in bursa; in spleen it was detected up to 14 DPI and in thymus and bone marrow up to 7 DPI. The IN strain vRNA was detected up to 21 DPI in bursa, 7 DPI in spleen and 3 DPI in thymus and bone marrow (Table; 2.13). In non-lymphoid tissues STC strain vRNA was detected in caecum up to 21DPI, pancreas, kidneys, lungs and liver up to 7 DPI. In thigh muscles it was detected up to DPI 3 and in breast muscles only one sample was detected positive at DPI 14. The IN strain vRNA was not detected in thigh muscles and was detected in breast muscles, pancreas, kidneys, caecum, lungs, liver up to DPI 7 (Table; 2.14). 39 Virus Isolation: Persistence of infectious virus was confirmed by inoculating the all RTPCR positive, bursal homogenate from SPF and commercial chickens (having maternally derived antibodies) in to chickens embryos. The STC virus was isolated up to 4 and 3 weeks PI from SPF and commercial chickens respectively. The IN strain was isolated up to 10 DPI from both SPF and commercial chickens (Figures; 2.2 A-D). ELISA: The SPF chickens were negative for antibodies before inoculation and IBDV specific antibodies were detected post-inoculation in STC and IN inoculated groups (Fig; 3.4AB). No antibodies were detected in virus free control SPF chickens during the experimental period. The antibodies titers were measured, in commercial broiler (having maternally derived antibodies and in ovo vaccinated chickens), data not shown. It was observed that at one day of age the antibody titer were high and at 2 weeks of age (at the time of inoculation) the antibodies titers declined to approximately 1/2 of the titers recorded at one day of age, data not shown. In brief, the overall persistence, distribution and vRNA load (approximately 2 grams/ tissue) was significantly different between SPF and commercial broiler chickens (Fig; 2.3). While comparing the persistence, distribution and vRNA load of the two strain used, the STC strain was significantly different than the IN strain (Fig; 2.1). 2.5 DISCUSSION This is the first comprehensive report on the persistence, distribution and quantification of viral RNA concentration of IBDV in SPF and commercial broiler chickens. We found that the classical strain STC, vRNA was detected longer than previously documented for 40 SPF and commercial broiler chickens. For both viral strains the highest vRNA concentration was found in the bursa followed by the caecum spleen, liver, kidney, pancreas, thymus, lungs, bone marrow, thigh muscles and breast muscles. This finding is particularly important in understanding IBDV pathogenesis. The persistence and distribution of IBDV strains was significantly different between SPF and commercial broiler chickens. Attempts were made to verify whether the RT-PCR positive results were indicative of the presence of infectious virus or viral RNA. The RT-PCR positive bursal homogenates (from SPF and commercial broilers having maternally derived antibodies) were inoculated to chickens embryos. The STC strain was isolated up to 28, DPI and 21 DPI from SPF and commercial chickens respectively. Whereas IN strain was isolated up to DPI 10, either from SPF or commercial chickens. When we inoculated 2 groups of commercial chickens (one group having maternal derived antibodies and the other was in ovo vaccinated) and compared them with SPF chickens (experiment 1 and 2), there was no significant difference in the persistence of virus in bursal tissues between the SPF and commercial chickens. Significant differences were determined in the persistence of virus/viral RNA in non-lymphoid tissues of SPF and commercial chickens. Previously in our laboratory(Abdel-Alim and Saif, 2001a), SPF chickens were inoculated with IBDV at 3 weeks of age, the viral RNA was detected by RT/PCR until 21 DPI, but attempts to isolate infectious virus from bursal homogenates failed. Infectious virus was detected by embryo inoculation up to 7 DPI in the bursa of SPF chickens inoculated at 2 or 3 weeks of age, whereas the viral RNA was detected by RT/PCR for up to DPI 28. In SPF chickens inoculated at 1 day of age, the 41 bursa-derived virus or its RNA was detected at DPI 7 and 14, when inoculated at a high 4 2.5 dose, 10 EID50/bird or at a low dose, 10 EID50/bird. In commercial 1-day-old broiler chickens, the bursa-derived virus was detected at 7 and 14 days PI when inoculated at a 4 high dose, 10 EID50/bird, whereas the virus was detected only at 14 days PI when inoculated at a low dose, 102.5 EID50/bird. In SPF and commercial chickens, vaccinated with a modified live IBDV vaccine, the virus is known to persist in the bursa of SPF chickens for 3 weeks but maternal antibodies in the commercial chickens readily eliminate it from the bursa since no live vaccine virus nor its RNA were detected in commercial broilers vaccinated at 1 day or 2 weeks of age (Abdel-Alim and Saif, 2001a). Previously Elankumaran et al., 2002, reported that a variant strain of IBDV was detected in virus-challenged commercial broilers for up to 6 weeks and infectious virus was reisolated from all tissues up to 4 weeks PI (Elankumaran et al., 2002). It is surprising to detect the viral RNA in SPF chickens up to DPI 56 and virus capable of replication could only be isolated from the bursal homogenates of the same experiment only up to DPI 28. It is possible that some of the RT-PCR positive samples only contained viral RNA and no infectious virus (Jackwood and Sommer-Wagner, 2010). It might be possible that virus persist in very low amount in the bursal cells that can be detected by RT-PCR but is not enough to establish successful infection when inoculated into chicken embryos or chickens. In a study it was shown that IBDV persistently infect the chicken B-lymphoid DT40 cells, a tumor cell line derived from the bursa of fabricius of a chicken infected with avian leukosis virus. Establishment of the persistent infection is associated with an 42 extensive remodeling of the hypervariable region of the VP2 capsid polypeptide, accumulating 14 amino acid changes during the first 60 days of the persistent infection. The amino acid sequence of the non-structural VP5 polypeptide, involved in virus dissemination, is not altered during the persistent infection (Delgui et al., 2009). There are reports that macrophages and monocytes can be infected with the virus (Kaufer and Weiss, 1976, 1980; Burkhardt and Muller, 1987; Komine et al., 1989; Inoue et al., 1992; Lam, 1998; Khatri et al., 2005; Palmquist et al., 2006). Chicken surviving IBDV infections are immunosuppressed despite repopulation of the bursa with B cells. It was shown that infection of neonatal chicks with a classical virulent IBDV strain (F52/70) causes severe bursal B cell depletion with recovery after about one week (Withers et al., 2005). In that study it was observed that two types of bursal follicles developed: normal large reconstituted follicles and small poorly developed follicles lacking a discernible cortex and medulla. It was demonstrated that the presence of large numbers of undifferentiated follicles was associated with inability to mount antibody responses to IBDV itself and after immunization with Salmonella enteritidis bacterin, indicating that B cells in these follicles are unable to produce peripheral B-cells with an effective immunoglobulin repertoire (Withers et al., 2005). Additionally, a number of inflammatory foci were observed in the recovering bursa. These foci contained few B cells at the margins, but large numbers of CD4+ and CD8+ cells, scattered T-cells and macrophages, and small central aggregates of dendritic like cells expressing the CD40 antigen (Withers et al., 2005). One might speculate the possibility that some of these cells may contain small amount of virus or viral antigen that can be detected by RT-PCR. It 43 will be interesting to further explore the possibility of the persistent infection of IBDV in chickens. Based on the findings in our persistence experiments we conclude that, thigh muscles and breast muscle were the least positive organs for virus/vRNA persistence. Hence we speculate that the persistence of IBDV in commercial chicken meat (breast and thigh muscles) is unlikely to be a major concern for international trade. The persistence and distribution of IBDV RNA in chickens depends on the presence of maternally derived antibodies. The presence of vRNA is not indicative of the presence of the infectious virus as indicated by virus isolation; hence virus isolation has to be performed to prove the presence of infectious virus. In addition, one has to be cautious in interpreting the results from experimental infection. We have shown earlier (Abdel-Alim and Saif, 2001a; Al-Natour et al., 2004), that inoculums and antibody titers are important determinants of virus persistence. Hence, results from experimental infections are at best a guide but not necessarily represents the field conditions. 2.6 ACKNOWLEDGEMENTS We acknowledge the salaries and research support provided by state and federal funds appropriated to the Ohio Agriculture Research and Development Center, The Ohio State University. We are grateful to Dr. Juliette Hanson, Gregory Myers and Kingsly Berlin for their help in animal work. We acknowledge Dr. Alexander Rodriguez-Palaci for statistical analysis of the data. 44 2.7 REFERENCES Abdel-Alim, G.A. and Saif, Y.M. (2001) Detection and persistence of infectious bursal disease virus in specific-pathogen-free and commercial broiler chickens. Avian Dis 45(3), 646-54. Al-Natour, M.Q., Ward, L.A., Saif, Y.M., Stewart-Brown, B. and Keck, L.D. (2004) Effect of diffferent levels of maternally derived antibodies on protection against infectious bursal disease virus. Avian Dis 48(1), 177-82. Anon. (1999) International Animal Health Code: mammals, birds and bees. Office International des Epizooties; 8th ed. Office International des Epizooties 8th ed. Ashraf, S., Abdel-Alim, G. and Saif, Y.M. (2006) Detection of antibodies against serotypes 1 and 2 infectious bursal disease virus by commercial ELISA kits. Avian Dis 50(1), 104-9. Australia. (2001. ) Generic import risk analysis (IRA) for uncooked chicken meat. IRA Report, 35. Delgui, L., Gonzalez, D. and Rodriguez, J.F. (2009) Infectious bursal disease virus persistently infects bursal B-lymphoid DT40 cells. J Gen Virol 90(Pt 5), 1148-52. Elankumaran, S., Heckert, R.A. and Moura, L. (2002) Pathogenesis and tissue distribution of a variant strain of infectious bursal disease virus in commercial broiler chickens. Avian Dis 46(1), 169-76. Henderson, K.S. and Jackwood, D.J. (1990) Comparison of the dot blot hybridization assay with antigen detection assays for the diagnosis of infectious bursal disease virus infections. Avian Dis 34(3), 744-8. Jackwood, D.J. and Sommer-Wagner, S.E. (2005) Molecular epidemiology of infectious bursal disease viruses: distribution and genetic analysis of newly emerging viruses in the United States. Avian Dis 49(2), 220-6. Jackwood, D.J. and Sommer-Wagner, S.E. (2010) Detection and characterization of infectious bursal disease viruses in broilers at processing. Prev Vet Med 97(1), 45-50. Luque, D., Rivas, G., Alfonso, C., Carrascosa, J.L., Rodriguez, J.F. and Caston, J.R. (2009) Infectious bursal disease virus is an icosahedral polyploid dsRNA virus. Proc Natl Acad Sci U S A 106(7), 2148-52. 45 Peters, M.A., Lin, T.L. and Wu, C.C. (2005) Real-time RT-PCR differentiation and quantitation of infectious bursal disease virus strains using dual-labeled fluorescent probes. J Virol Methods 127(1), 87-95. Rauf, A., Khatri, M., Murgia, M.V. and Saif, Y.M. (2011) Expression of perforingranzyme pathway genes in the bursa of infectious bursal disease virus-infected chickens. Dev Comp Immunol. Reed, L.J. and Muench, H.A. (1983) A simple method of estimation fifty per cent endpoint. . Am. J. Hyg 27, 493-497. Saif, Y.M. (2004) Control of infectious bursal disease virus by vaccination. Dev Biol (Basel) 119, 143-6. Wyeth, P.J. and Cullen, G.A. (1978) Susceptibility of chicks to infectious bursal disease (IBD) following vaccination of their parents with live IBD vaccine. Vet Rec 103(13), 281-2. Wyeth, P.J., O'Brien, J.D. and Cullen, G.A. (1981) Improved performance of progeny of broiler parent chickens vaccinated with infectious bursal disease oil-emulsion vaccine. Avian Dis 25(1), 228-41. 46 Table 2.1: Persistence and distribution of IBDV (STC & IN) strains in lymphoid tissues of two-weeks old SPF chickens IBDV genome detection by real time RT-PCR Thymus DPI* STC Bursa IN STC Spleen IN STC Bone marrow IN STC IN 47 3 3/3 (4.54±0.69)** 3/3 (5.08±0.48) 3/3 (7.12±0.86) 3/3 (9.13±0.12) 3/3 (4.97±0.91) 3/3 (5.54±0.57) 3/3 (4.46±0.47) 3/3 (4.98±0.48) 5 3/3 (4.38±0.12) 3/3 (4.45±0.47) 3/3 (5.75±0.95) 3/3 (6.33±0.84) 3/3 (4.22±0.72) 3/3 (4.26±0.51) 3/3 (1.96±0.69) 2/3 (3.46±0.47) 7 3/3 (4.57±0.73) 2/3 (3.78±0.02) 3/3 (5.91±0.32) 3/3 (5.4±00.15) 3/3 (4.35±0.46) 3/3 (3.16±0.19) 3/3 (1.98±0.50) 0/3 10 2/3 (3.3±0.43) 3/3 (3.57±0.76) 3/3 (5.60±0.25) 3/3 (5.71±1.04) 3/3 (3.19±0.12) 3/3 (2.66±0.74) 1/3 (1.26±00) 3/3 (6.98±0.76) 14 3/3 (2.81±0.13) 3/3 (2.75±0.49) 3/3 (5.38±0.30) 3/3 (4.68±0.44) 3/3 (3.57±0.41) 3/3 (2.97±0.2) 3/3 (0.79±0.14) 1/3 (6.78±00) 21 0/3 0/3 3/3 (4.08±0.25) 3/3 (4.25±0.22) 3/3 (0.78±0.17) 0/3 1/3 (0.68±00) 28 2/3 (1.21±0.57) 0/3 3/3 (3.54±0.36) 3/3 (3.41±0.61) 2/3 (2.2±0.04) 1/3 (2.43±00) 1/3 (1.39±00) 0/3 35 1/3 (1.87±00) 0/3 3/3 (3.53±0.63) 1/3 (3.86±00) 1/3 (1.31±00) 0/3 1/3 (0.65±00) 0/3 42 0/3 0/3 3/3 (3.17±0.61) 0/3 0/3 0/3 0/3 0/3 Dayinoculation post-inoculation **: positive/total Number positive/total inoculated (average log± 10 EID50/1ml ± Standard * DPI: DPI: Day post **: Number inoculated (average log 10 EID 50/50ul Standard deviation). 47 0/3 deviation). Table 2.2: Persistence and distribution of IBDV (STC & IN) strains in non-lymphoid tissues of two-weeks old SPF chickens IBDV genome detection by real time RT-PCR Thigh muscles DPI* STC Breast muscles IN STC Pancreas IN STC Kidney IN STC IN 48 3 3/3(3.92±0.90** 3/3 (3.22±0.78) 2/3 (2.69±0.014) 3/3 (3.02±0.32) 3/3 (3.85±0.17) 3/3 (3.45±1.14) 3/3 (4.09±0.89) 3/3 (3.71±0.47) 5 2/3 (2.01±0.50) 3/3 (1.84±0.15) 1/3 (2.61±00) 0/3 3/3 (3.26±0.47) 3/3 (2.42±0.67) 2/3 (3.17±0.84) 3/3 (2.84±0.82) 7 3/3 (1.85±0.54) 1/3 (1.69±00) 1/3 (1.94±00) 0/3 3/3 (3.09±0.73) 2/3 (2.63±0.65) 3/3 (3.42±0.38) 1/3(1.9±00) 10 1/3 (0.54±00) 1/3 (3.02±00) 1/3 (0.57±00) 3/3 (2.83±0.61) 3/3 (2.45±0.31) 0/3 2/3 (2.71±0.50) 2/3 (2.70±0.44) 14 2/3 (1.33±0.16) 1/3 (2.81±00) 2/3 (0.55±0.49) 2/3 (2.85±0.44) 3/3 (2.22±0.19) 1/3 (1.85±00) 3/3 (2.24±0.40) 3/3 (2.52±0.56) 21 1/3 (0.33±00) 0/3 1/3 (0.57±00) 0/3 2/3 (2.05±0.53) 0/3 2/3 (0.94±0.48) 0/3 28 2/3 (0.55±0.34) 0/3 0/3 0/3 1/3 (0.68±00) 0/3 3/3 (1.23±0.80) 0/3 35 0/3 0/3 1/3 (0.14±00) 0/3 2/3 (0.81±0.5) 0/3 1/3 (0.43.±00) 0/3 42 0/3 0/3 0/3 0/3 0/3 0/3 0/3 0/3 * DPI: DPI: Day post **: Number positive/total inoculated inoculated (average log(average 10 EID 50/50ul ± Standard deviation). Dayinoculation post-inoculation **: Number positive/total log 10 EID50/1ml ± Standard 48 deviation). Table 2.3 : Persistence and distribution of IBDV (STC&IN) in non-lymphoid tissues of two-weeks old SPF chickens IBDV genome detection by real time RT-PCR Caecum DPI* STC Lungs IN STC Liver IN STC IN 49 3 3/3 (4.63±0.73) ** 3/3 (5.2±0.53) 3/3 (3.46±1.93) 3/3 (3.37±0.93) 3/3 (4.31±1.21) 3/3 (5.69±0.25) 5 3/3 (4.68±0.40) 3/3 (4.47±0.51) 3/3 (3.55±0.71) 3/3 (2.92±0.78) 3/3 (4.43±0.32) 3/3 (4.5±0.95) 7 3/3 (3.21±1.13) 3/3 (3.95±0.02) 3/3 (3.11±0.08) 1/3 (1.67±00) 2/3 (3.71±0.26) 3/3 (2.99±0.33) 10 3/3 (2.08±1.25) 3/3 (2.7±0.12) 3/3 (1.95±0.73) 0/3 3/3 (2.05±0.80) 2/3 (2.97±0.33) 14 3/3 (1.75±0.62) 3/3 (2.59±0.37) 3/3 (1.29±0.29) 0/3 3/3 (2.39±0.65) 2/3 (1.56±0.87) 21 3/3 (0.83±0.49) 3/3 (2.81±1.5) 3/3 (0.69±0.14) 0/3 2/3 (1.92±0.40) 0/3 28 2/3 (1.86±0.12) 1/3 (4.6±00) 1/3 (0.51±00) 0/3 3/3 (1.42±0.10) 0/3 35 1/3 (1.66±0) 0/3 0/3 0/3 1/3 (2.23±00) 0/3 0/3 0/3 0/3 0/3 0/3 0/3 42 * DPI: Day post inoculation **: Number positive/total inoculated (average log 10 EID 50/50ul ± Standard deviation). DPI: Day post-inoculation **: Number positive/total inoculated (average log 10 EID50/1ml ± Standard deviation). 49 Table 2.4: Persistence and distribution of IBDV (STC & IN) strains in lymphoid tissues of four-weeks old SPF chickens IBDV genome detection by real time RT-PCR Thymus Bursa STC IN STC 7 2/3 (2.33±0.48)** 0/3 3/3 (4.38±0.59) 14 3/3 (1.45±0.24) 0/3 21 1/3 (1.44±00) 28 IN Bone marrow STC IN STC IN 2/3 (5.38±0.03) 3/3 (3.51±0.58) 3/3 (3.27±0.39) 3/3 (1.67±0.07) 1/3 (1.7±00) 3/3 (3.56±0.34) 3/3 (3.59±0.3) 3/3 (1.98±0.66) 2/3 (1.19±1.04) 0/3 0/3 0/3 3/3 (3.31±1.00) 3/3 (3.68±0.1) 3/3 (1.54±1.15) 1/3 (0.75±00) 1/3 (0.19±00) 0/3 2/3 (1.75±0.11) 0/3 3/3 (3.71±0.6) 3/3 (3.36±1.14) 2/3 (2.15±0.02) 0/3 1/3 (0.62±00) 0/3 35 3/3 (0.83±0.35) 0/3 3/3 (3.24±0.24) 0/3 3/3 (1.49±0.11) 0/3 0/3 0/3 42 0/3 0/3 2/3 (3.01±0.24) 0/3 2/3 (1.35±0.12) 0/3 0/3 0/3 49 0/3 0/3 2/3 (2.23±0.88) 0/3 1/3 (0.42±00) 0/3 1/3 (0.51±00) 0/3 56 0/3 0/3 2/3 (1.56±0.70) 0/3 1/3 (0.41±00) 0/3 0/3 0/3 50 DPI* Spleen DPI: DayDay post-inoculation Numberpositive/total positive/total inoculated (average EID /1ml ± Standard deviation). * DPI: post inoculation **: **: Number inoculated (average log 10 log EID10 50/50ul ±50Standard deviation). 50 Table 2.5: Persistence and distribution of IBDV (STC & IN) strains in non-lymphoid tissues of four-weeks old SPF chickens IBDV genome detection by real time RT-PCR Thigh muscles Breast muscles Pancreas Kidney 51 DPI* STC IN STC IN STC IN STC IN 7 1/3 (2.35±00)** 0/3 2/3 (1.5±0.42) 0/3 3/3 (2.24±0.15) 1/3 (2.25±00) 3/3 (2.4±0.38) 0/3 14 0/3 (00±00) 0/3 0/3 0/3 2/3 (1.19±0.28) 0/3 3/3 (2.15±0.53) 0/3 21 1/3 (0.47±00) 0/3 0/3 0/3 3/3 (1.48±0.79) 0/3 3/3 (0.95±0.46) 0/3 28 1/3 (0.092±00) 0/3 2/3 (0.83±0.47) 0/3 3/3 (1.62±0.44) 0/3 3/3 (1.85±0.32) 0/3 35 0/3 0/3 0/3 0/3 3/3 (1.03±0.11) 0/3 3/3 (1.18±0.19) 0/3 42 0/3 0/3 0/3 0/3 0/3 0/3 1/3 (1.6±00) 0/3 49 0/3 0/3 0/3 0/3 0/3 0/3 0/3 0/3 56 0/3 0/3 0/3 0/3 0/3 0/3 0/3 0/3 DPI: Day post-inoculation **:Number Number positive/total inoculated (average log50/50ul 10 EID Standard deviation). 50/1ml ±deviation). * DPI: Day post inoculation **: positive/total inoculated (average log 10 EID ± Standard 51 Table 2.6: Persistence and distribution of IBDV (STC&IN) in non-lymphoid tissues of four-weeks old SPF chickens IBDV genome detection by real time RT-PCR Caecum Lungs Liver 52 DPI* STC IN STC IN STC IN 7 3/3 (3.45±1.77)** 3/3 (3.03±0.29) 3/3 (1.7±0.76) 0/3 3/3 (3.87±3.01) 2/3 (2.78±0.014) 14 3/3 (2.76±0.85) 0/3 3/3 (3.5±3) 0/3 3/3 (3.1±2.54) 0/3 21 3/3 (1.7±0.37) 1/3 (2.00±00) 3/3 (3.7±0.78) 0/3 3/3 (3.2±0.12) 0/3 28 3/3 (2.3±0.16) 0/3 2/3 (3.4±1.66) 0/3 3/3 (3.1±0.47) 0/3 35 2/3 (0.95±0.08) 0/3 0/3 0/3 3/3 (3.44±2.15) 0/3 42 1/3 (1.04±00) 0/3 1/3 (38.20±0.48) 0/3 2/3 (3.6±1.39) 0/3 49 0/3 0/3 0/3 0/3 0/3 0/3 56 0/3 0/3 0/3 0/3 0/3 0/3 * DPI: Day post-inoculation **: Number positive/total inoculated (average log 10 EID50/1ml ± Standard deviation). 52 Table 2.7: Persistence and distribution of IBDV strains in lymphoid tissues of two-weeks old in ovo vaccinated broilers chickens IBDV genome detection by real time RT-PCR Thymus DPI* STC Bursa IN STC Spleen IN STC Bone marrow IN STC IN 53 3 0/3 (00±00)** 1/3 (2.95±00) 2/3 (4.90±1.24) 3/3 (5.64±1.71) 3/3 (1.87±0.37) 2/3 (3.57±0.57) 2/3 (0.71±0.42) 0/3 5 2/3 (1.94±0.12) 0/3 (00±00) 3/3 (4.28±1.02) 1/3 (4.23±00) 2/3 (1.30±0.23) 0/3 0/3 0/3 7 0/3 1/3 (4.23±00) 2/3 (5.44±0.57) 2/3 (4.89±0.92) 2/3 (1.24±0.51) 0/3 0/3 0/3 10 0/3 0/3 2/3 (4.75±0.12) 2/3 (4.05±0.55) 1/3 (1.91±00) 1/3 (2.31±00) 2/3 (0.98±0.05) 0/3 14 0/3 0/3 2/3 (0.71±0.88) 3/3 (3.23±0.83) 3/3 (3.61±0.76) 1/3 (1.60±00) 3/3 (1.46±0.68) 0/3 21 0/3 0/3 3/3 (3.47±0.76) 3/3 (3.35±0.86) 1/3 (1.80±00) 1/3 (6.95±00) 0/3 1/3 (5.67±00) 28 0/3 0/3 2/3 (0.93±0.15) 1/3 (3.26±00) 1/3 (0.78±00) 0/3 1/3 (1.99±00) 0/3 35 0/3 0/3 2/3 (1.39±0.60) 0/3 1/3 (0.59±00) 0/3 0/3 0/3 42 0/3 0/3 0/3 0/3 0/3 0/3 0/3 0/3 * DPI: Day post-inoculation **: Number positive/total inoculated (average log 10 EID50/1ml ± Standard deviation). 53 Table 2.8 : Persistence and distribution of IBDV strains in non-lymphoid tissues of two-weeks old in ovo vaccinated broilers IBDV genome detection by real time RT-PCR Thigh muscles DPI* STC Breast muscles IN STC Pancreas IN STC Kidney IN STC IN 54 3 1/3 (1.02±00)** 1/3 (3.21±00) 0/3 0/3 1/3 (0.51±00) 0/3 0/3 2/3 (3.29±0.79) 5 3/3 (1.78±0.17) 0/3 0/3 0/3 1/3 (0.54±00) 0/3 2/3 (0.49±0.62) 0/3 7 1/3 (1.47±00) 0/3 0/3 0/3 0/3 0/3 0/3 1/3 (2.64±00) 10 1/3 (0.56±00) 0/3 0/3 0/3 1/3 (0.56±00) 0/3 1/3 (1.61±00) 0/3 14 2/3 (1.33±0.16) 0/3 0/3 0/3 0/3 0/3 0/3 0/3 21 0/3 0/3 0/3 0/3 0/3 0/3 0/3 0/3 28 0/3 0/3 0/3 0/3 0/3 0/3 0/3 0/3 35 0/3 0/3 0/3 0/3 2/3 (1.31±0.42) 0/3 0/3 0/3 42 0/3 0/3 0/3 0/3 0/3 0/3 0/3 0/3 * DPI: Day post inoculation **: Number positive/total inoculated (average log 10 EID 50/1ml ± Standard deviation). 54 Table 2.9: Persistence and distribution of IBDV (STC & IN) strains in non-lymphoid tissues of two-weeks in ovo vaccinated broilers chickens IBDV genome detection by real time RT-PCR Caecum DPI* STC Lungs IN STC Liver IN STC IN 55 3 3/3 (0.92±0.14)** 3/3 (3.16±0.51) 0/3 0/3 1/3 (4.31±1.21) 3/3 (2.93±1.06) 5 2/3 (2.35±0.80) 1/3 (2.28±00) 1/3 (1.06±00) 0/3 2/3 (4.43±0.32) 0/3 7 2/3 (1.13±0..09) 1/3 (3.28±00) 1/3 (1.11±00) 0/3 0/3 0/3 10 3/3 (1.99±0.40) 0/3 1/3 (0.65±00) 0/3 2/3 (1.66±0.20) 0/3 14 1/3 (0.79±00) 0/3 2/3 (1.16±0.20) 0/3 0/3 0/3 21 1/3 (2.23±00) 2/3 (4.45±0.63) 0/3 0/3 1/3 (0.28±00) 0/3 28 1/3 (1.27±00) 0/3 0/3 0/3 1/3 (1.54±00) 0/3 35 1/3 (0.67±00) 0/3 0/3 0/3 1/3 (0.57±00) 0/3 42 0/3 (00±00) 0/3 0/3 0/3 0/3 0/3 * DPI: Day post-inoculation **: Number positive/total inoculated (average log 10 EID50/1ml ± Standard deviation). 55 Table 2.10: Persistence and distribution of IBDV (STC & IN) strains in lymphoid tissues of four-weeks in ovo vaccinated broilers chickens IBDV genome detection by real time RT-PCR Thymus DPI* STC Bursa IN STC Spleen IN STC Bone marrow IN STC IN 56 3 2/3 (2.61±1.40)** 1/3 (3.26±00) 3/3 (7.40±0.20) 2/3 (5.54±0.85) 3/3 (3.47±1.37) 1/3 (3.03±00) 1/3 (0.71±0.42) 0/3 7 1/3 (1.57±00) 2/3 (2.94±0.27) 1/3 (5.15±00) 3/3 (6.94±0.18) 2/3 (2.40±0.70) 3/3 (3.22±0.60) 1/3 (0.65±00) 0/3 14 0/3 (00±00) 0/3 3/3 (2.01±0.44) 2/3 (3.97±0.56) 2/3 (1.41±1.83) 0/3 1/3 (3.85±00) 0/3 21 2/3 (1.19±0.52) 0/3 3/3 (4.51±0.19) 3/3 (4.10±0.34) 3/3 (3.07±0.91) 1/3 (2.63±00) 1/3 (5.92±00) 0/3 28 2/3 (2.52±1.94) 1/3 (3.25±00) 3/3 (4.53±0.39) 3/3 (3.29±0.28) 2/3 (3.22±0.05) 0/3 1/3 (7.36±00) 0/3 35 1/3 (4.69±00) 1/3 (3.94±00) 3/3 (3.51±0.43) 3/3 (3.22±0.05) 0/3 0/3 0/3 0/3 42 1/3 (3.23±00) 0/3 2/3 (3.23±0.014) 2/3 (2.60±0.30) 0/3 0/3 0/3 0/3 * DPI: Day post-inoculation **: Number positive/total inoculated (average log 10 EID50/1ml ± Standard deviation). * DPI: Day post inoculation **: Number positive/total inoculated (average log 10 EID 50/50ul ± Standard deviation). 56 Table 2.11: Persistence and distribution of IBDV (STC & IN) strains in non-lymphoid tissues of four-weeks in ovo vaccinated broilers chickens IBDV genome detection by real time RT-PCR Thigh muscles DPI* STC Breast muscles IN STC Pancreas IN STC Kidney IN STC IN 1/3 (1.19±00)** 0/3 1/3 (1.33±00) 0/3 3/3 (2.1±1.06) 0/3 2/3 (2.57±1.70) 0/3 7 0/3 0/3 0/3 0/3 0/3 1/3 (1.60±00) 1/3 (0.36±00) 1/3 (1.53±00) 14 0/3 0/3 1/3 (1.41±00) 0/3 1/3 (1.99±00) 0/3 0/3 0/3 21 1/3 (6.63±00) 0/3 0/3 0/3 2/3 (1.27±0.42) 1/3 (7.25±00) 1/3 (1.94±00) 1/3 (7.98±00) 28 1/3 (4.54±00) 0/3 0/3 0/3 2/3 (4.14±1.14) 1/3 (7.04±00) 2/3 (4.47±0.49) 2/3 (8.67±2.78) 35 1/3 (7.09±00) 0/3 0/3 0/3 1/3 (4.69±00) 0/3 0/3 0/3 42 0/3 0/3 0/3 0/3 0/3 0/3 0/3 0/3 57 3 ** DPI: **:Number Numberpositive/total positive/total inoculated (average 10 EID 50/1ml ± Standard deviation). DPI: Day Day post-inoculation post inoculation **: inoculated (average log log 10 EID 50/50ul ± Standard deviation). 57 Table 2.12: Persistence and distribution of IBDV (STC & IN) strains in non-lymphoid tissues of four-weeks in ovo vaccinated broilers IBDV genome detection by real time RT-PCR Caecum DPI* STC Lungs IN STC Liver IN STC IN 58 3 3/3 (4.72±0.14)** 0/3 1/3 (1.95±0.5) 1/3 (2.±00) 2/3 (2.20±0.91) 3/3 (2.93±1.06) 7 2/3 (2.54±0.80) 3/3 (4.66±0.38) 1/3 (1.51±00) 2/3 (2.38±0.56) 2/3 (1.27±0.38) 0/3 14 1/3 (0.51±00) 1/3 (2.45±00) 0/3 (3.21±00) 0/3 0/3 0/3 21 3/3 (2.94±0.43) 1/3 (3.03±00) 2/3 (3.25±2.2) 0/3 0/3 0/3 28 3/3 (1.86±0.11) 1/3 (2.67±00) 3/3 (4.50±2.7) 0/3 2/3 (3.41±0.75) 0/3 35 3/3 (1.14±0.60) 0/3 0/3 0/3 0/3 0/3 42 0/3 0/3 0/3 0/3 0/3 0/3 * DPI: Day post-inoculation **: Number positive/total inoculated (average log 10 EID50/1ml ± Standard deviation). 58 Table 2.13: Persistence and distribution of IBDV strains in lymphoid tissues of 2-weeks old broilers having maternal antibodies IBDV genome detection by real time RT-PCR Thymus DPI* STC Bursa IN STC Spleen IN STC Bone marrow IN STC IN 2/3 (2.01±1.11)** 3/3 (4.18±0.5) 3/3 (6.16±0.58) 3/3 (7.19±0.40) 3/3 (2.88±0.88) 1/3 (3.72±00) 1/3 (0.88±00) 0/3 7 1/3 (1.23±00) 0/3 3/3 (5.15±1.17) 3/3 (5.2±0.56) 3/3 (3.54±0.80) 2/3 (3.66±0.1) 2/3 (1.03±0.22) 0/3 14 0/3 0/3 3/3 (4.81±0.39) 2/3 (4.43±0.56) 3/3 (1.21±0.76) 0/3 0/3 0/3 21 0/3 0/3 3/3 (4.16±0.21) 3/3 (2.87±0.6) 0/3 0/3 0/3 0/3 28 0/3 0/3 3/3 (2.59±1.15) 0/3 0/3 0/3 0/3 0/3 35 0/3 0/3 3/3 (2.80±0.66) 0/3 0/3 0/3 0/3 0/3 42 0/3 0/3 3/3 (2.28±2.8) 0/3 0/3 0/3 0/3 0/3 59 3 * DPI: Day post-inoculation **: Number positive/total inoculated (average log 10 EID50/1ml ± Standard deviation). 59 Table 2.14: Persistence and distribution of IBDV (STC & IN) strains in non-lymphoid tissues of 2-weeks broilers having maternal antibodies IBDV genome detection by real time RT-PCR Thigh muscles DPI* STC Breast muscles IN STC Pancreas IN STC Kidney IN STC IN 1/3 (1.26±00)** 0/3 0/3 0/3 1/3 (1.72±1.06) 0/3 1/3 (1.72±00) 1/3 (7.45±00) 7 0/3 0/3 0/3 2/3 (1.79±0.11) 2/3 (2.21±0.88) 1/3 (1.60±00) 2/3 (1.54±0.47) 1/3 (6.8±00) 14 0/3 0/3 1/3 (1.41±00) 0/3 0/3 0/3 0/3 0/3 21 0/3 0/3 0/3 0/3 0/3 0/3 0/3 0/3 28 0/3 0/3 0/3 0/3 0/3 0/3 0/3 0/3 35 0/3 0/3 0/3 0/3 0/3 0/3 0/3 0/3 42 0/3 0/3 0/3 0/3 0/3 0/3 0/3 0/3 60 3 * DPI: Day post **: Number positive/total inoculatedinoculated (average log 10 EID50log /50ul Standard * DPI: Dayinoculation post-inoculation **: Number positive/total (average 10±EID ± Standard deviation). 50/1ml deviation). 60 Table 2.15: Persistence and distribution of IBDV (STC & IN) strains in non-lymphoid tissues of 2-weeks broilers having maternal antibodies IBDV genome detection by real time RT-PCR Caecum DPI* STC Lungs IN STC Liver IN STC IN 61 3 3/3 (3.47±0.92)** 2/3 (3.62±0.38) 1/3 (1.95±0.5) 2/3 (2.69.±00) 3/3 (2.63±1.00) 2/3 (2.77±1.06) 7 3/3 (4.21±0.57) 2/3 (4.18±0.54) 1/3 (1.51±00) 2/3 (1.56±0.02) 3/3 (2.70±1.19) 1/3 (3.66±00) 14 1/3 (1.29±00) 0/3 0/3 0/3 0/3 0/3 21 3/3 (1.13±0.15) 0/3 0/3 0/3 0/3 0/3 28 0/3 0/3 0/3 0/3 0/3 0/3 35 0/3 0/3 0/3 0/3 0/3 0/3 42 0/3 0/3 0/3 0/3 0/3 0/3 * DPI: Day post-inoculation **: Number positive/total inoculated (average log 10 EID50/1ml ± Standard deviation). 61 Viral titers over time (all tissues) 3 5 7 10 14 21 28 35 42 49 56 42 49 56 42 49 56 IN 2 4 6 8 Indiana strain, n=792 0 CT–value value CTof LogLog titter 10 ofEID 10 Log / 1ml 10 50 3 5 7 10 14 21 28 35 STC 2 4 6 8 STC strain, n=810 0 log_with40 0 2 4 6 8 Non-inoculatedC controls, n=72 3 5 7 10 14 21 28 35 Days post inoculation Figure 2.1. Virus titer decay in tissue samples from chickens singly inoculated with reference strains of infectious bursal disease virus. STC virus was significantly more persistent overtime (GLM, p<0.001). The number of tissue samples tested is presented in italics (n=). 62 SPF chickens Log10 EID50/ 1ml (A) Commercial chickens 8 8 7 7 6 6 5 5 4 4 3 3 2 2 1 1 0 Log10 EID50/ 1mll (C) 8 7 14 21 28 42 49 56 8 7 6 6 5 5 4 4 3 3 2 2 1 8 1 0 7 0 7 14 21 28 35 42 days post-inoculation 6 bursa 2-week-old spf 2 IN Cecum: spf 4 IN 4-week-old 5 bursa 6 35 7 7 7 Virus isolated 0 0 80 8 (B) caecum spf 2 IN 49 56 0 7 14 (D) 21 28 35 42 49 56 bursa spf 2 IN 0 7 14 21 28 bursa spf 4 IN 42 49 56 35 bursa spf 2 IN days post-inoculation 6 caecum spf 2 IN bursa spf 4 IN 5 caecum spf 4 IN caecum spf 2 INT. muscle: 42-week-old thigh spf 2 IN 2-week-old 4-week-old caecum spf 4 IN thigh spf 4 IN 7 4-week-old 3 thigh spf 2 IN 4 Persistence of two strains of infectious 2 Figure 2.2. bursal disease virus after experimental caecum spf 4 IN thigh spf 4 IN 7 inoculation in SPF and commercial chickens. (A) Persistence of STC strain in SPF 3 thigh 1 spf 2 IN chickens. Virus isolation was confirmed in bursal homogenates which is represented by 4 IN 7 of STC strain0 in commercial chickens. Virus isolation was 2 thigh shaded line. (B) spf Persistence 21 28 of 35 42 49 56 confirmed1 up 21 DPI as shown by shaded0 line.7 (C) 14 Persistence IN strain in SPF chickens. Virus isolation was confirmed in bursal homogenates which is represented by 0 up to 10 DPI. (D) Persistence of IN strain in commercial chickens. Virus shaded line isolation was 10 DPI by shaded 0 confirmed 7 14up to21 28 as shown 35 42 49 line. 56 5 4 3 2 1 0 0 7 14 21 28 35 42 49 56 63 2 0 3 5 7 10 14 21 28 35 42 49 56 35 42 49 56 6 8 SPF Chickens 0 2 4 50 ul Log EID 50/ /1ml Log EID 10 10 50 log_with40 4 6 8 Commercial Commercialbroilers broilers 3 5 7 10 14 21 28 Figure 2.3. Log10 viral titers in experimentally inoculated SPF and commercial chickens. Data represents all tissues tested from animals infected with IN and STC strains of IBDV between 2 and 4 weeks of age (n=1,654 samples). Commercial broilers (2weeks-old) were studied until day 42 PI; SPF experiments were conducted until day 56 PI. 64 Antibody response aganist STC S/P (A) 1.4 1.2 1 0.8 0.6 0.4 0.2 0 0 10 20 30 40 50 DPI Antibody response aganist IN S/P (B) 2 1.8 1.6 1.4 1.2 1 0.8 0.6 0.4 0.2 0 0 10 20 30 40 50 D.P.I Figure 2.4. Antibody titer in SPF chickens after inoculation with STC and IN strains. Antibodies titers were measured by IDDEX kit and observed O.D values were converted in to S/P ratio as per manufacturer instructions. (A) Antibody titer in STC challenged chickens and (B) Antibody titer in IN strain challenged chickens. 65 CHAPTER 3 DIFFERENTIAL MODULATION OF CYTOKINES AND TOLL LIKE RECEPTORS EXPRESSION IN THE BURSA OF CHICKENS INFECTED WITH CLASSICAL AND VARIANT STRAINS OF INFECTIOUS BURSAL DISEASE VIRUS. 3.1 SUMMARY Infectious bursal disease (IBD) is an important immunosuppressive disease of chickens. The causative agent, infectious bursal disease virus (IBDV), consists of two serotypes, 1 and 2. Serotype 1 consists of classic IBDV (cIBDV) and variant IBDV (vIBDV). Both of these strains vary in antigenicity and pathogenicity. The goal of this study was to compare the immunopathogenesis of cIBDV and vIBDV. Three-weeks-old specific pathogen free chickens were inoculated intraocularly with standard challenge strain (STC) (cIBDV) and a variant strain Indiana (IN) (vIBDV). The cIBDV produced more pronounced bursal damage and inflammatory response as compared to vIBDV. There were significant differences in the expression of innate (IFN-α and IFN-β), proinflammatory cytokine and mediator (IL-6 and iNOS) in cIBDV- and vIBDV-infected bursas. The expression of genes of chemokines, IL-8 and MIP-α was also higher in 66 cIBDV -infected chickens compared to control during the early phase of infection. The expression of Toll like receptor 3 (TLR3) was downregulated at PIDs 3, 5, and 7 in the bursas of vIBDV-infected chickens whereas TLR3 was upregulated at PIDs 3 and 5 in cIBDV-infected bursas. In vIBDV-infected bursa, TLR7 expression was downregulated at PIDs 3 and 5 and upregulated at PID 7. However, TLR7 was upregulated at PIDs 3 and 7 in cIBDV-infected bursas. The expression of MyD88 was downregulated whereas TRIF gene expression was upregulated in cIBDV- and vIBDV-infected bursa. These findings will be useful in understanding the differential immuno-pathogenesis of classical and variant strains of IBDV. 3.2 INTRODUCTION Infectious bursal disease (IBD) is one of the most important naturally occurring viral diseases of commercial chickens worldwide (Lukert P.D., 2003). The causative agent, IBD virus (IBDV) belongs to the family Birnaviridae. The virus causes an acute, highly contagious and immunosuppressive disease in chickens (Lukert P.D., 2003). The virus infects and destroys actively dividing IgM-bearing B cells in the bursa of fabricius (Hirai et al., 1981; Rodenberg et al., 1994). IBDV exists in different antigenic and pathogenic forms (Jackwood et al., 2006). Initial isolates designated as classical strains (cIBDV) of the serotype 1 viruses were considered to be a single antigenic type. In the early 1980s, antigenic variants (vIBDV) of the virus were identified in the United States (Lukert P.D., 2003). These variant viruses were able to cause disease in the presence of immunity to cIBDV viruses (Ismail and Saif, 1991). The antigenic variants typically do not cause clinical signs of disease but can cause a marked immunosuppression (Jackwood et al., 67 2006). The immunosuppression caused by variants and classical strains of IBDV is often associated with secondary viral respiratory infections and bacterial infections (Bautista et al., 2004; Li et al., 2010; Subler et al., 2006). The IBDV induced immunosuppression also renders chicken flocks refractory to live attenuated vaccines against other viral diseases such as avian influenza virus, infectious bronchitis and Newcastle disease virus (Muller et al., 2003; Ramirez-Nieto et al., 2010). Following infection and replication of IBDV, T cells infiltrate the bursa of infected chickens (Kim et al., 2000; Sharma et al., 2000). Although B cells are considered the major targets for IBDV, it has been shown that the virus can infect and possibly replicate in macrophages (Khatri et al., 2005; Kim et al., 1998; Palmquist et al., 2006). Following viral infections, including IBDV, activated macrophages produce various mediators, such as proinflammatory cytokines, interleukin-1 (IL-1) and IL-6, chemokines, and nitric oxide (NO) (Glass et al., 2003; Heitmeier et al., 1998; Khatri et al., 2005; Khatri and Sharma, 2006). Host cells use various receptors to detect viral infections by recognizing pathogenassociated molecular patterns (PAMPs) and subsequently induce an antiviral response. Prominent among these are Toll-like receptors (TLRs) (Kawai and Akira, 2006; Sang et al., 2008; Werts et al., 2006). Several TLRs recognize viral PAMPs: TLR3, detects double-stranded RNA (dsRNA) derived from viral replication whereas single-stranded RNA (ssRNA) are detected by TLR7 and TLR8 (Sang et al., 2008). The TLR signaling proceeds via two pathways; the myeloid differentiation factor 88 (MyD88)-mediated pathway and the Toll-interleukin-1 receptor (TIR)-domain-containing adaptor inducing 68 IFN-β (TRIF)-mediated pathway (Kawai and Akira, 2009; Takeuchi and Akira, 2009). The TLR signaling pathways arise from intracytoplasmic TIR domains, which are conserved among all TLRs. The TLR7 specifically involves MyD88-dependent pathway, whereas TRIF is implicated in the TLR3-mediated MyD88-independent pathway (Takeda and Akira, 2004). The IBD is controlled by vaccination and the vaccines are very effective against classical strains but with the emergence of variant and the very virulent strains of IBDV in the United States (Stoute et al., 2009), there are several incidents of vaccine failure (Berg and Meulemans, 1991; Ismail et al., 1990; Okoye and Shoyinka, 1983; Snyder, 1990; Stoute et al., 2009). This highlights the need to examine the differential immuno-pathogenesis of classical and variant strains of IBDV in order to devise better control strategies. In this study, for the first time, we examined the differential immuno-pathogenesis of classical and variant strains of IBDV. As compared to vIBDV, cIBDV induced early bursal lesions, extensive infiltration of T cells in the bursa and induced higher expression of proinflammatory cytokine and mediators; IL-6 and iNOS. Further, there were differences in the expression of TLR3 and TLR7 and their adapter molecules, TRIF and MyD88, in the bursa of cIBDV and vIBDV-infected chickens. Elucidation of the TLRs signaling pathway and factors leading to activation of the innate immune response to IBDV infection may provide new strategies for the development of cross-protective vaccines against IBDV. 69 3.3 MATERIALS AND METHODS Chickens and virus strains: Specific pathogen free (SPF) chicken eggs (Charles River Laboratories Inc; Wilmington, MA, USA) were incubated and hatched in our facility at The Ohio Agricultural Research and Development Center, The Ohio State University. Chickens were kept in a disease containment building. At 3-weeks of age, prior to inoculation with virus, the chickens were transferred to an isolation unit. The standard challenge strain, (STC) (Abdel-Alim and Saif, 2001a) representing cIBDV strain, and a variant Indiana (IN) representing (vIBDV), were propagated in chickens and titrated in eggs as described earlier (Ismail et al., 1990). Experimental design: Eighty-four SPF chickens were allocated to 3 groups; thirty-six chickens in both group 1 and group 2 were inoculated intraocularly with 104 EID50/200ul of either vIBDV or cIBDV strains respectively and 12 chickens in group 3 were inoculated similarly with PBS to serve as virus-free controls (Abdel-Alim and Saif, 2001b). At post-inoculation days (PIDs) 3, 5 and 7, twelve chickens each from the virus-infected groups and 4 chickens from the virus-free group were euthanized and bursas were collected. Four pools of 3 bursas each from virus-infected groups were prepared. The harvested bursal tissues were examined for the following: 1- histopathological lesions, 2- immunohistochemical detection of virus antigen, T cells and macrophages, 3- isolation of mononuclear cells and expression of virus-induced innate, proinflammatory cytokines, 70 chemokines, Toll like receptors (TLRs), and their adaptor molecules by quantitative real time RT-PCR (qRT-PCR). Microscopic lesions: At PIDs 3, 5 and 7, four bursas each from virus-free control, cIBDV- and vIBDVinfected chickens were harvested, fixed in 10 % phosphate buffered formalin, sectioned and stained with hematoxylin and eosin for the detection of histopathological lesions. Bursal follicular lesions were observed microscopically and lesion scores were determined. The bursal lesions were scored as follows: lesion score 1 represents 1-25% of lymphoid follicles affected, 2 represents 26-50% of lymphoid follicles affected, 3 represents 51-75% of lymphoid follicles affected and 4 represents 76-100% of lymphoid follicles affected (Kim et al., 1999; Rautenschlein et al., 2007). Detection of IBDV-antigen, macrophages and T cells in virus-infected bursas: The IBDV antigens, T cells, and macrophages were detected in snap frozen sections of bursas of vIBDV-, cIBDV-infected and virus-free control chickens by immunohistochemistry (Khatri et al., 2005; Rauf et al., 2011). Briefly, small sections of bursal tissue were embedded in Tissue-Tek® O.C.T compound (Sakura Finetek, CA, USA), sectioned 5 μM thick with the help of cryostat microtome (Leica CM 1510S Germany), spotted on double positive glass slides, and dehydrated at 37 oC overnight. The dehydrated sections were fixed with acetone (75% acetone and 25% ethyl alcohol) for 10 minutes followed by 3 washes with phosphate buffered saline (PBS). The tissue sections were then blocked with 2% goat serum for 1 hour (hr). After blocking, the sections were incubated with respective primary and secondary antibodies. A biotin71 streptavidin-peroxidase method using R.T.U vectastain(R) kit (Vector Laboratories, Burlingame, CA) was adopted for the detection of viral antigen, T cells and macrophages in frozen sections of vIBDV-, cIBDV-infected and virus-free chickens bursas. The primary antibodies used for the detection of T cells and macrophages were: mouse antichicken CD3, (diluted 1:200) and mouse anti-chicken monocytes/macrophage KUL01, (diluted 1:400) (Southern Biotech, Birmingham, AL, USA). The primary antibody used for the detection of IBDV antigen was biotinylated mouse anti-IBDV polyclonal antibody (diluted 1:100). The development of dark brown color indicated a positive reaction. The group means ± SEM of vIBDV- and cIBDV-infected cells, macrophage or T cells per field was determined at 20 X magnification after counting 5 fields /bursa/ chicken and compared with virus-free control groups. Isolation of bursal mononuclear cells: Twelve bursas either from cIBDV- or vIBDV-infected groups at each PID were pooled into 4 pools of three bursas each and bursas were also collected from 4 virus-free control chickens at each PID. Mononuclear cells were isolated from bursas as previously described (Khatri et al., 2005; Rauf et al., 2011). Briefly, mononuclear cells suspension was prepared from bursas by density gradient centrifugation (gradient density 1.090) over Ficoll-Hypaque (GE healthcare Bio-Sciences Uppsala Sweden) and washed twice in cold RPMI 1640 (Gibco, Carlsbad, CA). The cell pellets were lysed with Trizol reagent and stored at -70 for RNA extraction. 72 RNA extraction and qRT-PCR: Total RNA from bursal mononuclear cells of virus-infected and virus-free control chickens was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. qRT-PCR was used for the quantification of genes specific for the expression of messenger RNAs (mRNAs) for innate (IFN-α, IFN-β) and proinflammatory (IL-6 and iNOS) cytokines, chemokines (IL-8 and MIP-α), Toll like receptors and their adaptor molecules (TLR3, TLR7, MyD88 and TRIF) (Khatri et al., 2005). The primers for 28S, IFN-α, IFN-β, IL-6, iNOS, IL-8, MIP-α, TLRL3, TLR7, TRIF and MyD88 were designed according to previously published sequences (Hghihghi et al., 2010; Jarosinski et al., 2002; Sijben et al., 2003). RT-PCR was performed using Power SYBER® Green RNA – to-CT TM 1 step RT-PCR kit (Applied Biosystems, Foster City, CA). Amplification and detection were performed in an automated 7500 Real time RT-PCR system (Applied Bio System, Foster City, CA). Fold increase of target gene expression over uninfected controls was calculated with the 2-ΔΔCT method (Giulietti et al., 2001; Khatri et al., 2005; Palmquist et al., 2006; Rauf et al., 2011). Statistical analysis: Graph Pad Prism version 5 for Windows was used for graphical presentation of data. Student’s t-test was used to detect significant differences between vIBDV- and cIBDVinfected chickens. P < 0.05 was considered to be statistically significant. 73 3.4 RESULTS Infection of chickens with cIBDV and vIBDV: Inoculation of chickens with vIBDV and cIBDV resulted in a typical IBDV infection. All of the chickens inoculated with cIBDV showed morbidity and 8% mortality whereas mortality was not observed in chickens infected with vIBDV. Virus-free control chickens had no clinical signs or macroscopic lesions during the course of the experiment. Histologically, cIBDV induced early and more pronounced bursal damage as compared to vIBDV (Fig. 1A). Severe follicular lesions were observed histologically in the bursa of cIBDV-infected chickens at PID 3 with a mean bursal lesions score of 3.25 ± 0.50 whereas in vIBDV-infected group, the lesion score at PID 3 was 1.75 ±0.50. At PIDs 5 and 7, the lesion score increased to 3.75 ± 0.50 and 4 ± 00 respectively in cIBDVinfected bursa. In vIBDV-infected bursa, lesion score was 3.25 ± 0.50 and 3.75 ± 0.50 at PIDs 5 and 7, respectively (Fig. 3.1B). Detection of IBDV-antigen, T cells and macrophages in virus-infected bursa: We detected viral antigen, T cells and macrophages in cIBDV- and vIBDV-infected bursa at PID 3, 5 and 7 by immunohistochemistry. Number of IBDV antigen positive cells was significantly higher (P<0.05) in cIBDV-infected bursa as compared to bursa of vIBDV-infected chickens at all the PIDs tested (Fig. 3.2A and B). Similar to viral antigen positive cells, we observed significantly higher (P<0.05) number of infiltrating T cells in the bursa of cIBDV-infected chickens as compared to vIBDV-infected chickens at PID 3 and 5 (Fig. 3.3A and B). However, both viral strains induced infiltration of similar number of macrophages in the bursa which was maximum at PID 5 (Fig. 3.4A and B). 74 These results indicate that cIBDV replicated extensively in the bursa and induced more pronounced infiltration of T cells as compared to vIBDV. Virus induced expression of innate cytokines: Both vIBDV and cIBDV induced innate cytokine response in virus infected chicken bursas. The innate cytokine IFN-α was upregulated at PID 3, 5 and 7 in the bursa of vIBDV-infected chickens (Fig. 3.5A). In cIBDV-infected chickens, IFN-α was downregulated at PID 3 and upregulated at PID 5 and 7 (Fig. 3.5A). IFN-β was upregulated in cIBDV- and vIBDV-infected chickens at PIDs 3, 5 and 7 (Fig. 3.5B). Expression of proinflammatory cytokine, mediator and chemokines in IBDVinfected bursa: Classical strains of IBDV are known to induce a strong inflammatory response in the bursa. In this study, we detected stronger upregulation in the gene expression of IL-6 in the bursa of cIBDV-infected chickens than vIBDV-infected chickens during the early stage of infection (Fig. 3.6A). Similarly, cIBDV induced significantly higher (P<0.05) expression of iNOS as compared to vIBDV at PID 3 (Fig. 3.6B). Expression of the chemokine IL-8 was also higher in cIBDV-infected bursa at PID 3 (Fig. 3.6C). MIP-α expression was significantly elevated (P<0.05) in cIBDV-infected chickens at PID 3 and substantially elevated at PID 7 as compared to vIBDV-infected chickens (Fig. 3.6D). These data suggest that both viral strains activate proinflammatory cytokine and chemokine responses which are more pronounced in cIBDV-infected chickens. 75 Expression of toll like receptors and adaptor molecules in virus-infected bursa: The gene expression data of TLRs and their adaptor molecules are illustrated in Fig. 3.7. We noted downregulation in the expression of TLR3 in vIBDV-infected bursa at all PIDs tested (Fig. 3.7A). However, in cIBDV-infected bursa, TLR3 gene expression was significantly upregulated at PID 3 and 5 as compared to vIBDV, and it was downregulated at PID 7 (Fig. 3.7A). The expression level of TLR 7 showed a general trend of downregulation during the early stage of infection in chickens infected with either viral strain (Fig. 3.7B). Expression of TRIF was upregulated in both cIBDV- and vIBDV-infected chickens at all PIDs (Fig. 3.7C). The gene expression of MyD88 in vIBDV-infected bursas was downregulated at all time points. MyD88 was upregulated at PID 3 and downregulated at PID 5 and 7 in cIBDV-infected chickens (Fig. 3.7D). 3.5 DISCUSSION Innate immune responses orchestrate the antiviral activities through the proliferation of different effector cells such as macrophages, T-cells and effector molecules such as cytokines, chemokines and Toll like receptors. In this study, while comparing the pathogenesis of cIBDV and vIBDV, we noted that cIBDV produced more pronounced bursal damage as compared to vIBDV during early stages of the infection. Both viral strains induced pronounced infiltration of macrophages and T cells in the bursa of infected chickens. The expression of the proinflammatory cytokines and chemokines in cIBDV-infected bursa was significantly higher than vIBDV-infected bursa. Importantly, 76 we observed that cIBDV infection upregulated the expression of TLR3 whereas vIBDV infection downregulated its expression. Following infection, IBDV replicates extensively in B cells and causes bursal atrophy (Sharma et al., 2000). This study revealed that there were distinct differences in the early pathogenic events of the classical and variant strains of IBDV. Although both viruses caused bursal atrophy and lymphoid cell depletion, cIBDV replicated extensively in the bursa and induced more bursal lesions. Bursal lesions in cIBDV-infected bursa were accompanied by infiltration of inflammatory cells and well pronounced plical edema. In contrast, bursal lesions induced by vIBDV were accompanied by thickening of the intra follicular septum and a less marked inflammatory response. We also noted the inhibition of the expression of the antiviral cytokine IFN-α in cIBDV-infected chickens early in the infection. The lack of IFN-α early in the infection would provide an opportunity for the virus to establish an infection. In a previous study (Eldaghayes et al., 2006), inhibition of IFN-α in virulent IBDV-infected chickens was also observed. Although antibody mediated immunity plays an important role, it has been demonstrated that cell mediated immunity is also crucial against IBDV infection. This study compared for the first time the infiltration of T cells and macrophages in cIBDV- and vIBDVinfected bursa. The infiltration of T cells was significantly higher (P<0.05) in cIBDVinfected bursa during the early stages of the infection. Previously, it was shown that a virulent strain of IBDV induced extensive infiltration of T cells as compared to an avirulent strain (Rautenschlein et al., 2003). In our study, T cell infiltration was higher in cIBDV-infected chickens and cIBDV appeared to be more virulent (based on bursal 77 lesions score). These findings indicate that infiltration of T cells may be related to the virulence of the virus strain. The T cells are hypothesized to mediate the virus clearance and may also be responsible for the exacerbated bursal lesions (Rautenschlein et al., 2002a). Higher infiltration of T cells in cIBDV-infected chickens may have contributed to the enhanced bursal damage observed during the early stage of the infection. Although not tested in this study, both classical and variant strains caused functional impairment of T cells and T cells responded poorly to mitogens in vitro (Sharma et al., 1989). Macrophages are the central effector cells of the innate immune system. Cytokines produced by innate immune cells influence the nature of the adaptive immune response (Mogensen et al., 2004). We observed the recruitment of macrophages in cIBDV- and vIBDV-infected bursa. Previously, we have shown that macrophages from IBDVinfected chickens produce proinflammatory cytokine (Khatri et al., 2005). In the present study, expression of the proinflammatory cytokine IL-6 and mediator iNOS was significantly upregulated in cIBDV-infected chickens as compared to vIBDV-infected chickens. The increased expression of IL-6 and iNOS correlated well with the previous reports where based on histological observations, it was shown that variant IBDV induced mild inflammatory response as compared to classical IBDV (Ismail and Saif, 1991; Sharma et al., 1989). The mechanisms responsible for the recruitment of macrophages to the bursa of IBDV-infected chickens are not known. However, it is likely that the chemokines, IL-8 and MIP-α, may have a role in this regard. Chicken IL-8 acts as a chemoattractant for heterophils and monocytes (Barker et al., 1993). In this study, we found that the expression of these chemokines was significantly upregulated in cIBDV78 infected birds and slightly upregulated in vIBDV-infected birds. Although the number of macrophages in cIBDV- and vIBDV-infected bursa was the same, cytokine and chemokine data suggest that macrophages were likely to be more activated in cIBDVinfected chickens. Activated macrophages in chickens are known to be a source of proinflammatory cytokines (Khatri et al., 2005; Palmquist et al., 2006). The TLRs have been established to play a pivotal role in the activation of innate immunity by recognizing specific patterns of microbial components. In the present study, we demonstrate for the first time, the induction of TLRs and their adaptor proteins, TRIF and MyD88 in IBDV-infected chickens. TLR3 and TLR7 are the only TLRs implicated in antiviral responses in chickens (Hghihghi et al., 2010). Strikingly, TLR3 was downregulated in vIBDV-infected bursa whereas it was upregulated in cIBDV-infected bursa. In addition to TLR3, melanoma differentiation-associated gene 5 (MDA5) and retinoic acid inducible gene I (RIG-I) are also receptors for dsRNA (Andrejeva et al., 2004; Yoneyama et al., 2004). The MDA5 and RIG-1 activate IRF3 and NF-κB for the induction of innate immunity. Previously, we have shown that NF-κB regulates IBDVinduced cytokine production (Khatri and Sharma, 2006). Therefore, it is possible that vIBDV induced activation of the innate response may be MDA5/RIG1 mediated. Previously, TLR3 upregulation was shown in chickens infected with very virulent MDV and H5N1 avian influenza infection (Abdul-Careem et al., 2009; Karpala et al., 2008). The expression of TLR3 adaptor protein TRIF was upregulated in cIBDV- and vIBDVinfected bursa. Further in vitro studies using a siRNA approach will be needed to 79 delineate the role of TRIF-dependent or independent TLR3/MDA5/RIG1-mediated innate immune activation by cIBDV and vIBDV. While examining the expression of TLR7 in infected bursas, we found that TLR7 gene expression was downregulated in vIBDV-infected bursa. However, in cIBDV-infected bursas TLR7 gene expression was upregulated. TLR7 primarily acts as a receptor for ssRNA; however, dsRNA duplexes of 19–21 bp in length can activate mammalian TLR7 (Heil et al., 2004; Ward et al., 2005). It is possible that the dsRNA genome of IBDV may activate avian TLR7. In our study, MyD88 was downregulated in cIBDV- and vIBDVinfected bursa. Previously, it was shown that dsRNA-triggered, TLR3-mediated signaling is independent of MyD88 (Jiang et al., 2004). In conclusion, this study reports striking differences in the pathogenesis and activation of host responses by cIBDV and vIBDV. Compared to vIBDV, cIBDV produced more pronounced bursal damage, accumulation of T cells, and inflammatory response (IL-6 and iNOS expression). Further studies are needed to identify the role of viral proteins responsible for mediating the differential host responses against classical and variant IBDV and the cellular source of cytokines and chemokines produced in the bursa. The findings of this study provide new insights that could be useful for the design of effective vaccines against classical and variant IBDV strains. 3.6 ACKNOWLEDGEMENTS We acknowledge the salaries and research support provided by state and federal funds appropriated to the Ohio Agriculture Research and Development Center, The Ohio State University. We are grateful to Dr. Juliette Hanson, Gregory Myers and Kingsly Berlin for 80 their help in animal work. We acknowledge Dr. Michele Williams for critically reviewing the manuscript. 3.7 REFERENCES Abdel-Alim, G.A. and Saif, Y.M. (2001a) Detection and persistence of infectious bursal disease virus in specific-pathogen-free and commercial broiler chickens. Avian Dis 45(3), 646-54. Abdel-Alim, G.A. and Saif, Y.M. (2001b) Immunogenicity and antigenicity of very virulent strains of infectious bursal disease viruses. Avian Dis 45(1), 92-101. Abdul-Careem, M.F., Haq, K., Shanmuganathan, S., Read, L.R., Schat, K.A., Heidari, M. and Sharif, S. (2009) Induction of innate host responses in the lungs of chickens following infection with a very virulent strain of Marek's disease virus. Virology 393(2), 250-7. Andrejeva, J., Childs, K.S., Young, D.F., Carlos, T.S., Stock, N., Goodbourn, S. and Randall, R.E. (2004) The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci U S A 101(49), 17264-9. Barker, K.A., Hampe, A., Stoeckle, M.Y. and Hanafusa, H. (1993) Transformationassociated cytokine 9E3/CEF4 is chemotactic for chicken peripheral blood mononuclear cells. J Virol 67(6), 3528-33. Bautista, D.A., Elankumaran, S. and Heckert, R.A. (2004) Effect of a variant infectious bursal disease virus (E/Del) on Salmonella typhimurium infection in commercial broiler chickens. Avian Dis 48(2), 361-9. Berg, T.P. and Meulemans, G. (1991) Acute infectious bursal disease in poultry: protection afforded by maternally derived antibodies and interference with live vaccination. Avian Pathol 20(3), 409-21. Eldaghayes, I., Rothwell, L., Williams, A., Withers, D., Balu, S., Davison, F. and Kaiser, P. (2006) Infectious bursal disease virus: strains that differ in virulence differentially modulate the innate immune response to infection in the chicken bursa. Viral Immunol 19(1), 83-91. Giulietti, A., Overbergh, L., Valckx, D., Decallonne, B., Bouillon, R. and Mathieu, C. (2001) An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25(4), 386-401. 81 Glass, W.G., Rosenberg, H.F. and Murphy, P.M. (2003) Chemokine regulation of inflammation during acute viral infection. Curr Opin Allergy Clin Immunol 3(6), 467-73. Heil, F., Hemmi, H., Hochrein, H., Ampenberger, F., Kirschning, C., Akira, S., Lipford, G., Wagner, H. and Bauer, S. (2004) Species-specific recognition of singlestranded RNA via toll-like receptor 7 and 8. Science 303(5663), 1526-9. Heitmeier, M.R., Scarim, A.L. and Corbett, J.A. (1998) Double-stranded RNA-induced inducible nitric-oxide synthase expression and interleukin-1 release by murine macrophages requires NF-kappaB activation. J Biol Chem 273(24), 15301-7. Hghihghi, H.R., Read, L.R., Mohammadi, H., Pei, Y., Ursprung, C., Nagy, E., Behboudi, S., Haeryfar, S.M. and Sharif, S. (2010) Characterization of host responses against a recombinant fowlpox virus-vectored vaccine expressing the hemagglutinin antigen of an avian influenza virus. Clin Vaccine Immunol 17(3), 454-63. Hirai, K., Funakoshi, T., Nakai, T. and Shimakura, S. (1981) Sequential changes in the number of surface immunoglobulin-bearing B lymphocytes in infectious bursal disease virus-infected chickens. Avian Dis 25(2), 484-96. Ismail, N.M. and Saif, Y.M. (1991) Immunogenicity of infectious bursal disease viruses in chickens. Avian Dis 35(3), 460-9. Ismail, N.M., Saif, Y.M., Wigle, W.L., Havenstein, G.B. and Jackson, C. (1990) Infectious bursal disease virus variant from commercial Leghorn pullets. Avian Dis 34(1), 141-5. Jackwood, D.J., Cookson, K.C., Sommer-Wagner, S.E., Le Galludec, H. and de Wit, J.J. (2006) Molecular characteristics of infectious bursal disease viruses from asymptomatic broiler flocks in Europe. Avian Dis 50(4), 532-6. Jarosinski, K.W., Yunis, R., O'Connell, P.H., Markowski-Grimsrud, C.J. and Schat, K.A. (2002) Influence of genetic resistance of the chicken and virulence of Marek's disease virus (MDV) on nitric oxide responses after MDV infection. Avian Dis 46(3), 636-49. Jiang, Z., Mak, T.W., Sen, G. and Li, X. (2004) Toll-like receptor 3-mediated activation of NF-kappaB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-beta. Proc Natl Acad Sci U S A 101(10), 3533-8. Karpala, A.J., Lowenthal, J.W. and Bean, A.G. (2008) Activation of the TLR3 pathway regulates IFNbeta production in chickens. Dev Comp Immunol 32(4), 435-44. Kawai, T. and Akira, S. (2006) Innate immune recognition of viral infection. Nat Immunol 7(2), 131-7. 82 Kawai, T. and Akira, S. (2009) The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol 21(4), 317-37. Khatri, M., Palmquist, J.M., Cha, R.M. and Sharma, J.M. (2005) Infection and activation of bursal macrophages by virulent infectious bursal disease virus. Virus Res 113(1), 44-50. Khatri, M. and Sharma, J.M. (2006) Infectious bursal disease virus infection induces macrophage activation via p38 MAPK and NF-kappaB pathways. Virus Res 118(1-2), 70-7. Kim, I.J., Gagic, M. and Sharma, J.M. (1999) Recovery of antibody-producing ability and lymphocyte repopulation of bursal follicles in chickens exposed to infectious bursal disease virus. Avian Dis 43(3), 401-13. Kim, I.J., Karaca, K., Pertile, T.L., Erickson, S.A. and Sharma, J.M. (1998) Enhanced expression of cytokine genes in spleen macrophages during acute infection with infectious bursal disease virus in chickens. Vet Immunol Immunopathol 61(2-4), 331-41. Kim, I.J., You, S.K., Kim, H., Yeh, H.Y. and Sharma, J.M. (2000) Characteristics of bursal T lymphocytes induced by infectious bursal disease virus. J Virol 74(19), 8884-92. Li, G., Lillehoj, H.S., Lee, K.W., Jang, S.I., Marc, P., Gay, C.G., Ritter, G.D., Bautista, D.A., Phillips, K., Neumann, A.P., Rehberger, T.G. and Siragusa, G.R. (2010) An outbreak of gangrenous dermatitis in commercial broiler chickens. Avian Pathol 39(4), 247-53. Lukert P.D., S., Y.M.,, ed. (2003) Infectious bursal disease. 11th ed. Diseases of Poultry. Edited by B. Saif Y.M., H.J., Glisson, J.R., Fadly, A.M., McDougald, L.R., Swayne, D.E. . Iowa State University Press, Ames. Mogensen, T.H., Melchjorsen, J., Malmgaard, L., Casola, A. and Paludan, S.R. (2004) Suppression of proinflammatory cytokine expression by herpes simplex virus type 1. J Virol 78(11), 5883-90. Muller, H., Islam, M.R. and Raue, R. (2003) Research on infectious bursal disease--the past, the present and the future. Vet Microbiol 97(1-2), 153-65. Okoye, J.O. and Shoyinka, S.V. (1983) Newcastle disease in a vaccinated flock which had experienced subclinical infectious bursal disease. Trop Anim Health Prod 15(4), 221-5. Palmquist, J.M., Khatri, M., Cha, R.M., Goddeeris, B.M., Walcheck, B. and Sharma, J.M. (2006) In vivo activation of chicken macrophages by infectious bursal disease virus. Viral Immunol 19(2), 305-15. 83 Ramirez-Nieto, G., Shivaprasad, H.L., Kim, C.H., Lillehoj, H.S., Song, H., Osorio, I.G. and Perez, D.R. (2010) Adaptation of a mallard H5N2 low pathogenicity influenza virus in chickens with prior history of infection with infectious bursal disease virus. Avian Dis 54(1 Suppl), 513-21. Rauf, A., Khatri, M., Murgia, M.V. and Saif, Y.M. (2011) Expression of perforingranzyme pathway genes in the bursa of infectious bursal disease virus-infected chickens. Dev Comp Immunol. Rautenschlein, S., von Samson-Himmelstjerna, G. and Haase, C. (2007) A comparison of immune responses to infection with virulent infectious bursal disease virus (IBDV) between specific-pathogen-free chickens infected at 12 and 28 days of age. Vet Immunol Immunopathol 115(3-4), 251-60. Rautenschlein, S., Yeh, H.Y., Njenga, M.K. and Sharma, J.M. (2002) Role of intrabursal T cells in infectious bursal disease virus (IBDV) infection: T cells promote viral clearance but delay follicular recovery. Arch Virol 147(2), 285-304. Rautenschlein, S., Yeh, H.Y. and Sharma, J.M. (2003) Comparative immunopathogenesis of mild, intermediate, and virulent strains of classic infectious bursal disease virus. Avian Dis 47(1), 66-78. Rodenberg, J., Sharma, J.M., Belzer, S.W., Nordgren, R.M. and Naqi, S. (1994) Flow cytometric analysis of B cell and T cell subpopulations in specific-pathogen-free chickens infected with infectious bursal disease virus. Avian Dis 38(1), 16-21. Sang, Y., Ross, C.R., Rowland, R.R. and Blecha, F. (2008) Toll-like receptor 3 activation decreases porcine arterivirus infection. Viral Immunol 21(3), 303-13. Sharma, J.M., Dohms, J.E. and Metz, A.L. (1989) Comparative pathogenesis of serotype 1 and variant serotype 1 isolates of infectious bursal disease virus and their effect on humoral and cellular immune competence of specific-pathogen-free chickens. Avian Dis 33(1), 112-24. Sharma, J.M., Kim, I.J., Rautenschlein, S. and Yeh, H.Y. (2000) Infectious bursal disease virus of chickens: pathogenesis and immunosuppression. Dev Comp Immunol 24(2-3), 223-35. Sijben, J.W., Klasing, K.C., Schrama, J.W., Parmentier, H.K., van der Poel, J.J., Savelkoul, H.F. and Kaiser, P. (2003) Early in vivo cytokine genes expression in chickens after challenge with Salmonella typhimurium lipopolysaccharide and modulation by dietary n--3 polyunsaturated fatty acids. Dev Comp Immunol 27(67), 611-9. Snyder, D.B. (1990) Changes in the field status of infectious bursal disease virus. Avian Pathol 19(3), 419-23. 84 Stoute, S.T., Jackwood, D.J., Sommer-Wagner, S.E., Cooper, G.L., Anderson, M.L., Woolcock, P.R., Bickford, A.A., Senties-Cue, C.G. and Charlton, B.R. (2009) The diagnosis of very virulent infectious bursal disease in California pullets. Avian Dis 53(2), 321-6. Subler, K.A., Mickael, C.S. and Jackwood, D.J. (2006) Infectious bursal disease virusinduced immunosuppression exacerbates Campylobacter jejuni colonization and shedding in chickens. Avian Dis 50(2), 179-84. Takeda, K. and Akira, S. (2004) TLR signaling pathways. Semin Immunol 16(1), 3-9. Takeuchi, O. and Akira, S. (2009) Innate immunity to virus infection. Immunol Rev 227(1), 75-86. Ward, J.R., Bingle, L., Judge, H.M., Brown, S.B., Storey, R.F., Whyte, M.K., Dower, S.K., Buttle, D.J. and Sabroe, I. (2005) Agonists of toll-like receptor (TLR)2 and TLR4 are unable to modulate platelet activation by adenosine diphosphate and platelet activating factor. Thromb Haemost 94(4), 831-8. Werts, C., Girardin, S.E. and Philpott, D.J. (2006) TIR, CARD and PYRIN: three domains for an antimicrobial triad. Cell Death Differ 13(5), 798-815. Yoneyama, M., Kikuchi, M., Natsukawa, T., Shinobu, N., Imaizumi, T., Miyagishi, M., Taira, K., Akira, S. and Fujita, T. (2004) The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 5(7), 730-7. 85 Table 3.1. Sequence of primers for quantitative real-time RT-PCR analysis Target sequence Primers Reference 28 S IL-6 iNOS IL-8 IFN-α IFN-β TLR3 TRIF TLR7 MyD88 Forward 5-GGCGAAGCCAGAGGAAACT-3 Reverse 5-GACGACCGATTTGCACGTC-3 Forward 5-GCTCGCCGGCTTCGA-3 Reverse 5-GGTAGGTCTGAAAGGCGAACAG-3 Forward 5-GAGTGGTTTAAGGAGTTGGATCTGA3 Reverse 5-TTCCAGACCTCCCACCTCAA-3 Forward 5-GCCCTCCTCCTGGTTTCA G-3 Reverse 5-TGGCACCGCAGCTCATT-3 Forward 5-GACAGCCAACGCCAAAGC Reverse 5-GTCGCTGCTGTCCAAGCATT Forward 5-CCTCCAACACCTCTTCAACATG Reverse 5-TGGCGTGCGGTCAAT Forward 5-TCAGTACATTTGTAACACCCCGCC-3 Reverse 5-GGCGTCATAATCAAACACTCC-3 Forward 5-GCTGACCAAGAACTTCCTGTGC-3 Reverse 5-AGAGTTCTCATCCA AGGCCACC-3 Forward 5-TTCTGGCCACAGATGTGACC-3 Reverse 5-CCTTCAACTTGGCAGTGCAG-3 Forward 5-AGAAGGTGTCGGAGGATGGT-3 Reverse 5-GCTGGATGCTATTGCCTCGCTG-3 86 (Sijben et al., 2003). (Sijben et al., 2003). (Jarosinski et al., 2002). (Sijben et al., 2003). (Eldaghayes et al., 2006) (Eldaghayes et al., 2006). (Hghihghi et al., 2010). (Hghihghi et al., 2010). (Hghihghi et al., 2010). (Hghihghi et al., 2010). Fig.3.1: Histopathological lesions in bursa following IBDV-infection. (A) bursa of virus free control chickens at PIDs 3, 5 and 7 (a, b and c; 20 X); bursa of vIBDV-infected chickens at PIDs 3, 5 and 7 (d, e and f; 20 X); bursa of cIBDV-infected chickens at 3, 5 and 7 PID (g, h and I; 20 X) (note mild to moderate follicular depletion in d, e, and f and severe follicular depletion in g, h and i). (B) Bursal lesions score of follicular depletion in virus free control, vIBDV and cIBDV-infected chickens. * indicates statistically significant differences between vIBDV- and cIBDV-infected groups (p < 0.05). 87 (A) vIBDV Virus free cIBDV d g b e h c f i PID 7 PID 5 PID 3 a (B) 5 PID 3 Lesion score 4 PID 5 * PID 7 3 2 1 0 virus free vIBDV-Infected cIBDV-Infected Figure 3.1. Histopathological lesions in bursa following IBDV-infection. 88 Fig.3.2: IBDV antigen detection by immunohistochemical staining in virus-infected bursa. SPF chickens were inoculated with 104EID50 of cIBDV or vIBDV and bursal tissues were collected at PIDs 3, 5 and 7. Bursal sections from virus free chickens (a, b and c; 20X), vIBDV-infected chickens (d, e, and f; 20X) and cIBDV-infected chickens (g, h and I; 200X) were examined for the presence of IBDV antigen by immunohistochemistry using mouse anti IBDV antibody. Brown color indicated by arrow represents the positive staining. (B) IBDV positive cells were counted (40X) at PID 3, 5 and 7. The values represent the mean ±SEM of 5 fields/bursa/chicken on designated PID. * indicates statistically significant differences between vIBDV- and cIBDV-infected groups (p < 0.05). 89 (A) Virus free d g PID 3 a cIBDV vIBDV e c f h PID 5 b PID 7 i Mean Antign+cells / microscopic field (B) 200 * PID 3 PID 5 150 * PID 7 * 100 50 0 vIBDV-infected cIBDV-infected Figure 3.2. IBDV antigen detection by immunohistochemical staining in virus-infected bursa. 90 Fig.3.3: T cells infiltration in the IBDV-infected bursa. SPF chickens were inoculated with 104EID50 of cIBDV or vIBDV and bursal tissues were collected at PID 3, 5 and 7. Bursal sections from virus free chickens (a, b and c; 20X), vIBDV-infected bursa (d, e, and f; 20X) and cIBDV-infected bursa (g, h, and i; 20X) were examined for the presence of T cells by immunohistochemistry using anti-chicken CD3+ monoclonal antibody. Brown color indicated by arrow represents the positive staining. (B) T cells were counted (40X) at PID 3, 5 and 7. The values represent the mean ±SEM of 5 fields/bursa/chicken on designated PID. * indicates statistically significant differences between vIBDV- and cIBDV-infected groups (p < 0.05). 91 (A) cIBDV vIBDV Virus-free d gg b e h PID 5 PID 3 a c i PID 7 f (B) Mean Tcells / microscopic field 200 * PID 3 150 PID 5 PID 7 * 100 50 0 Virus-free vIBDV-infected cIBDV-infected Figure 3.3. T cells infiltration in the IBDV-infected bursa. 92 Fig. 3.4: Infiltration of macrophages in the IBDV-infected bursa. SPF chickens were inoculated with 104EID50 of cIBDV or vIBDV and bursal tissues were collected at PID 3, 5 and 7. Bursal sections from virus free chickens (a, b and c; 20X), vIBDV-infected chickens (d, e, and f; 20X) and cIBDV-infected chickens (g, h, and i; 20X) were examined for the presence of macrophages by immunohistochemistry using anti-chicken macrophage antibody. Brown color indicated by arrow represents the positive staining. (B) Macrophage positive cells were counted (20X) at PIDs 3, 5 and 7. The values represent the mean ±SEM of 5 fields/bursa/chicken on designated PID. * indicates statistically significant differences between vIBDV- and cIBDV-infected groups (p < 0.05). 93 (A) vIBDV Virus free cIBDV d g b e h PID 5 PID 3 a (f) c f PID 7 i Mean Macs +cells / microscopic field (B) 100 PID 3 75 PID 5 PID 7 50 25 0 control group vIBDV-infected cIBDV-infected Figure 3.4. Infiltration of macrophages in the IBDV-infected bursa. 94 Fig.4.5: Relative gene expression at mRNA level, of innate cytokines mRNA in IBDVinfected bursa. At PIDs 3, 5 and 7, bursal mononuclear cells were isolated from cIBDVor vIBDV-infected and virus-free control chickens and examined for (A) IFN-α and (B) IFN-β gene expression by qRT-PCR. Results are shown as transcription of the target gene relative to housekeeping gene 28S. The data is expressed as fold change expression in infected chickens over virus-free control. The values represent the mean ±SE of 4 pools of 3 bursa each at designated PID. * indicates statistically significant differences between cIBDV- and vIBDV -infected groups (p < 0.05). 95 Fig. 5. (A) Relative fold change of IFN- 10 -10 PID 3 -30 PID 5 PID 7 -50 -70 * vIBDV-infected cIBDV-infected (B) Relative fold change of IFN- 8 PID 3 PID 5 6 PID 7 4 * 2 0 vIBDV-infected cIBDV-infected Figure 3.5. Relative gene expression at mRNA level, of innate cytokines in IBDVinfected bursa. 96 Fig.3.6: Relative gene expression at mRNA level, of proinflamatory cytokines, iNOS and chemokines, mRNA in IBDV-infected bursa. At PIDs 3, 5 and 7, bursal mononuclear cells were isolated from cIBDV, vIBDV and virus-free control chickens and examined for (A) IL-6 (B) iNOS, (C), IL-8 and (D) MIP-α gene expression by qRT-PCR. Results are shown as transcription of the target gene relative to housekeeping gene 28S. The data is expressed as fold change expression in infected chickens over virus-free control. The values represent the mean ±SE of 4 pools of 3 bursa each at designated PID. * indicates statistically significant differences between vIBDV and cIBDV virus infected groups (p < 0.05). 97 Figure 3.6. Relative gene expression at mRNA level, of proinflamatory cytokines, iNOS and chemokines, in IBDV-infected bursa. 98 Fig. 3.7: Relative gene expression at mRNA level, of Toll like receptors and adaptor molecules mRNA in IBDV-Infected bursa. At PIDs 3, 5 and 7, bursal mononuclear cells were isolated from cIBDV, vIBDV and virus-free control chickens and examined for (A) TLR3, (B) TLR7, (C) TRIF and (D) MyD88 gene expression by qRT-PCR. Results are shown as transcription of the target gene relative to housekeeping gene 28S. The data is expressed as fold change expression in infected chickens over virus-free control. The values represent the mean ±SE of 4 pools of 3 bursa each at designated PID. * indicates statistically significant differences between vIBDV- and cIBDV-infected groups (p < 0.05). 99 Figure 3.7. Relative gene expression at mRNA level, of Toll like receptors and adaptor molecules in IBDV-Infected bursa. 100 CHAPTER 4 EXPRESSION OF PERFORIN-GRANZYME PATHWAY GENES IN THE BURSA OF INFECTIOUS BURSAL DISEASE VIRUS-INFECTED CHICKENS 4.1 SUMMARY Infectious bursal disease (IBD) is an economically important immunosuppressive disease of chickens. The IBD virus (IBDV) actively replicates in B cells and causes severe bursal damage. Generally, T cells are refractory to infection with IBDV but are known to promote virus clearance. However, the mechanisms of T cell mediated viral clearance are not well understood. In this study, we evaluated the molecular mechanisms of cytotoxic T cell responses in the pathogenesis of IBD in chickens. Infection of chickens with IBDV was accompanied by the infiltration of CD4+ and CD8+ T cells in the bursa. There was an upregulation in the gene expression of important cytolytic molecules; perforin (PFN), granzyme-A (Gzm-A), DNA repair and apoptotic proteins; high mobility proteins group (HMG) and poly (ADP-ribose) polymerase (PARP) in the bursa of Fabricius (BF) whereas expression of NK (natural killer) lysin was downregulated. Importantly, PFN 101 producing CD4+ and CD8+ T cells were also detected in the bursa of IBDV-infected chickens by immunohistochemistry. The Th1 cytokines, IL-2 and IFN-γ expression was also strongly upregulated, suggesting the activation of T cells. The findings of this study highlight the mechanisms of IBD pathogenesis and the role of cytotoxic T cells in the clearance of virus-infected cells. 4.2 INTRODUCTION Infectious bursal disease is an important immunosuppressive viral disease of chickens which causes substantial economic losses to poultry producers around the world. The IBD virus belongs to the family Birnaviridae and has a polyploid, bisegmented genome. The virus preferentially replicates in IgM-bearing B cells in the bursa of Fabricius (BF). The acute phase of the disease lasts for about 7 to 10 days. During this phase, bursal follicles are depleted of B cells and the BF becomes atrophic (Sharma et al., 2000). A strong antibody response against IBDV is considered important in defense against virulent virus. However, it is well established that antibody alone is not sufficient to provide complete protection. T cells rapidly infiltrate the BF starting at an early stage of virus infection (Tanimura and Sharma, 1997). Colocalization of T cells with replicating virus suggested that T cells may be involved in the host defense. Also, chickens rendered deficient in antibody production by chemical bursectomy recover fully from infection and develop an anamnestic cellular immune response (Rautenschlein et al., 2002b). Studies with surgical and chemical thymectomy have indicated that inactivated IBDV also requires T cell involvement (Yeh et al., 2002). 102 protection by Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells play central roles in the immune response against virus-infected or transformed cells. Cytotoxic cells kill target cells by releasing a membrane-disrupting protein known as perforin (PFN) and granzymes (Gzms) secretion by exocytosis and induce apoptosis of the target cell (Smyth and Trapani, 1995; Trapani and Smyth, 2002). The granule-exocytosis pathway activates cell death through either caspase-dependent or caspase-independent pathways (Sarin et al., 1997; Trapani et al., 1998; Trapani and Smyth, 2002). Another well recognized mechanism of cell cytotoxicity which functions independent of PFN is the engagement of Fas ligand triggering the caspase-dependent pathway of apoptosis (Nagata and Golstein, 1995; Van Parijs and Abbas, 1996). In the granule exocytosis pathway, Gzm-A causes DNA damage through activation of the SET (endoplasmic reticulum-associated) complex. The cleavage of the nucleosome assembly protein SET and the DNA-binding protein, high mobility group-2 (HMG-2) by Gzm-A interferes with DNA replication, repair, and transcription. Poly (ADP-ribose) polymerase (PARP) which primarily regulates the caspase-dependent cell lysis pathway, also interacts with the SET complex and depletes the cellular energy stores at the time of DNA fragmentation to further facilitate apoptosis (Mullbacher et al., 1999; Trapani and Smyth, 2002) . Not much is known about the mechanisms of cytotoxic T cell responses against IBDV in chickens. Previously, gene expression of Gzm-A and PFN has been reported in Marek’s disease virus (MDV)-infected chickens (Sarson et al., 2008). In this study we have demonstrated the infiltration of T cell subsets in the BF, gene expression of PFN, Gzm-A and molecules involved in DNA repair and apoptosis and the presence of PFN producing 103 CD4+ and CD8+ T cells in IBDV-infected bursa. Importantly, IL-2 and IFN-γ were also detected in cells isolated from IBDV-infected bursa suggesting that activated T cells may be involved in antiviral immunity and mediation of virus clearance from the BF. The findings of this study will help in understanding the role of T cells in the pathogenesis of IBD and may help in the design of better vaccines effective against both classical and variant IBDV. 4.3 MATERIALS AND METHODS Chickens and virus: Specific pathogen free (SPF) chicken eggs (Charles River Laboratories Inc. Wilmington, MA, USA) were incubated and hatched at our facilities at The Ohio Agriculture Research and Development Center, The Ohio State University. The chickens were kept in a disease containment building that had rooms supplied with HEPA filtered intake and exhaust air. At 3-weeks of age chickens were transferred to hard sided isolators supplied with HEPA filters and exhaust air. The classical IBDV strain STC (Abdel-Alim and Saif, 2001a) maintained in our laboratory was used to infect chickens. Experimental design: Forty-eight 3 weeks-old chickens were allotted into 2 groups; 12 chickens were used as virus free controls and 36 chickens were inoculated intraocularly each with 104 embryo infectious dose50 (EID50)/200 µl of IBDV (Abdel-Alim and Saif, 2001b). At post-inoculation days (PIDs) 3, 5 and 7, twelve chickens from virus infected group and 4 chickens from the virus free group were euthanized and BF was collected. Bursas were examined for the following: (a) gross lesions, (b) quantification of viral load by quantitative real time RT-PCR (qRT-PCR) and viral genome detection by RT-PCR, 104 (c) isolation of mononuclear cells and expression of PFN, Gzm-A, HMG, PAPR and, IL2 and IFN-γ gene by qRT-PCR and (d) detection of T cell subsets and PFN by immunohistochemistry. Isolation of bursal mononuclear cells: Twelve bursa collected at each PID were divided into 4 pools of three bursa each from infected chickens. BF was also collected from 4 virus-free control chickens at each PID. Mononuclear cells were isolated from the BF as previously described (Khatri et al., 2005; Pertile et al., 1995). Briefly, a mononuclear cells suspension was prepared from BF by density gradient centrifugation over FicollHypaque (GE healthcare Bio-Sciences Uppsala Sweden) (gradient density 1.090) and washed twice in cold RPMI 1640 (Gibco, Carlsbad). The cell pellet was lysed by adding Trizol reagent (Invitrogen, Carlsbad, CA) and stored at -70 OC for RNA extraction. RNA extraction and qRT-PCR: Total RNA from bursal mononuclear cells of IBDVinfected and virus free control chickens was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Quantitative RTPCR (qRT-PCR) was used to examine the gene expression of PFN, Gzm-A, HMG, PARP, NK lysin, IL-2 and IFN-γ using previously published gene specific primers (Eldaghayes et al., 2006; Sarson et al., 2008). Two-fold dilutions of total RNA were used to standardize gene amplification. Fifty nanograms of total RNA was found to be optimal and used throughout. qRT-PCR was performed using Power SYBER Green RNA – toCT 1 step RT-PCR kit (Applied Bio System, Foster City CA). Amplification and detection were performed in an automated 7500 Real time RT-PCR system (Applied bio system, Foster city CA) (Khatri et al., 2005). 105 Quantitation of the mRNA was determined by the comparative cycle threshold (CT) method. Bursal mononuclear cells from virus-free control chickens were used as the reference sample (calibrator) at each time point. The relative change in mRNA concentration (∆CT) for each test sample was then determined from the difference between the calibrator CT and the CT of each test sample. Each sample was run in duplicate. Fold increase of 28S, PFN, Gzm-A, HMG, PARP and NK lysin transcript expression over uninfected controls was calculated with the 2--∆∆CT method (Giulietti et al., 2001; Khatri et al., 2005; Palmquist et al., 2006). RT-PCR to detect IBDV genome: Total RNA from bursal mononuclear cells of IBDVinfected and virus free control chickens was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. One step RTPCR kit AgPath-IDTM (Applied bio system, Foster city CA) was used for the amplification of IBDV genome using previously published primers and probe (Peters et al., 2005). Serial tenfold dilutions of known tittered IBDV-STC strain were used to generate the standard curve and CT values of the test samples were used to estimate EID50 values. Detection of T cell subsets and PFN in IBDV-infected BF: The T cells subsets, CD4+ and CD8+ T cells and PFN were detected in frozen sections of virus-free and IBDVinfected bursal tissues by immunohistochemistry (Brewer et al., 2004; Khatri et al., 2005). Briefly small pieces of bursal tissue were embedded in tissues– Tek O.C.T compound (Sakura Finetek, CA, USA) using cryomold standard disposable vinyl specimen molds (25 mm x 20mm x 5mm) (Sakura Finetek, CA, USA) and stored at -70 106 o C for 30 minutes to obtain optimal cutting temperature for frozen sections. The frozen sections were cut to a thin layer of tissue (5 μm thick) with the help of cryostat microtome (Leica CM 1510S) and spotted on double positive glass slides and dehydrated at 37 oC overnight. The dehydrated sections were fixed with chilled acetone (75% acetone and 25% ethyl alcohol) for 5 minutes at -20 OC followed by 3 washing steps with PBS (phosphate buffered saline). The tissues sections were then blocked with 2 % goat serum for 1 hour. The primary and secondary antibodies used for the detection of CD4+ and CD8+ T cells were: mouse anti-chicken CD4 and CD8α at 1:200 dilutions (Southern Biotech, Birmingham, AL, USA) and biotinylated goat anti-mouse IgG (H+L) 1:500 dilution (Vector laboratories, Burlingame, CA). After washing, the sections were incubated with ABC reagent (Vector laboratories, Burlingame, CA) for 30 min. The primary and secondary antibodies used for the detection of PFN staining were: polyclonal rabbit anti-human PFN antibody (1:50) (BioVision research products, CA, USA) and goat polyclonal anti rabbit IgG (H+L)–HRP at 1:400 dilutions (Abcam Inc; Cambridge MA, USA). The reaction was developed with 3-3′diaminobenzidine (DAB) substrate. The dark brown chromogens detected on the tissues were considered as positive CD4+ and CD8+ T cells and PFN cells respectively. The immunostained sections were evaluated using Olympus 1X 70 microscope (Olympus Optical Co., Ltd., Tokyo, Japan). The number of CD4+ and CD8+T cells and PFN positive cells were counted as previously described (Rautenschlein and Haase, 2005). Briefly the group means of the numbers of T cells and PFN-positive cells were determined per microscopic fields, at a magnification of 20X after counting 5 fields/BF/bird. 107 PFN producing CD4+ or CD8+ T cells in the bursa were detected by double staining (Brewer et al., 2004). In double staining, PFN staining was developed with DAB (brown color) and CD4+ or CD8+ T cells staining was developed by incubating the sections with commercial Vector-SG peroxidase substrate kit (grey blue color) (Vector laboratories, Burlingame, CA). Statistical analysis: Student’s t test was used to detect significant differences between virus-free and IBDV-infected chickens. P<0.05 was considered to be statistically significant. 4.4 RESULTS Response of chickens to IBDV: Inoculation of chickens with IBDV resulted in a typical IBDV infection. Two chickens out of 36 in IBDV-inoculated group died at PID 3. The IBDV infection was confirmed by gross bursal lesions at necropsy and by virus quantification and detection of viral genome in the BF (Fig. 1A and B). Based on a standard curve developed for known titered IBDV, the CT values obtained from qRTPCR were converted into log10 EID50. The virus titer in BF at PID 3, 5 and 7 was 6.59 ± 0.16, 6.2 ± 0.51 and 6.02 ± 0.37 EID50/50μl (Fig. 4.1A). Expression of PFN, Gzm-A and molecules involved in DNA repair and apoptosis in bursal mononuclear cells: The gene expression of PFN, Gzm-A, HMG and PARP in bursal mononuclear cells was detected by qRT-PCR. Expression of PFN was upregulated early in the infection at PIDs 3 and 5 and was downregulated (3 fold) at PID 7 (Fig. 4.2A), whereas upregulated expression of Gzm-A was detected at all PIDs tested and was at peak at PID 7 (Fig. 2B). Similarly, HMG gene expression was upregulated at PIDs 3, 5 108 and 7 with peak upregulation at PID 5 (75 fold) (Fig. 4.2 C). PARP gene expression was downregulated early at PID 3 (3 fold) whereas it was 20- and 34- fold up regulated at PID 5 and 7, respectively (Fig. 4.2 D). The gene expression of NK lysin was 30-, 40- and 45fold downregulated at PIDs 3, 5, and 7 respectively (Fig. 4.2 E). Detection of CD4+, CD8+ T cells and PFN in IBDV-infected BF: At PIDs 3, 5 and 7, T cell subsets; CD8+ and CD4+ T cells and PFN were detected in the BF of IBDV-infected chickens by immunohistochemistry (Figs. 3A, 4A and 5A). The highest numbers of CD8+ cells were found on PID 7 (44.8 ± 9.8) whereas at PIDs 3 and 5 the numbers of CD8+ positive cells were 39.7 ± 13.5 and 33.7 ± 11.8, respectively (Figure 3 B). The highest numbers of CD4+ cells were found at PID 3 (37.2 ± 3.1) whereas at PIDs 5 and 7 the numbers of CD4+ positive cells were 28 ± 3.1 and 22.8 ± 5.1, respectively (Fig. 4.4 B). The highest numbers of PFN positive cells were found on PID 7 (24.9 ± 3.6) whereas at PIDs 3 and 5 the numbers of PFN positive cells were 16.2 ± 5.5 and 24.6 ± 3.1, respectively (Fig. 4.5 D). We also detected the PFN producing CD4+ and CD8+ T cells by double staining in IBDV-infected bursa (Fig. 4.5 B and 4.5 C). Expression of Th-1 cytokines IBDV-infected BF: To examine whether the infiltrating T cells in the bursa are activated, we detected the expression of Th-1 cytokines in bursal cells by qRT-PCR. Infection with IBDV resulted in increased mRNA expression of IL-2 and IFN-γ in bursal cells (Fig 4.6). The IL-2 gene expression at PIDs 3 and 5 was upregulated whereas at PID 7 it was downregulated (Fig. 4.6A). The accumulation of IFN-γ mRNA was detectable at PID 3 which further increased at PID 5 and 7. At PID 7, 109 bursal cells from IBDV-inoculated chickens had approximately 200-fold higher levels of IFN-γ mRNA than bursal cells from control birds (Fig. 4.6B). 4.5 DISCUSSION Elimination of invading pathogens requires a coordinated effort between effector lymphocytes for containment and clearance of virus-infected cells (Trapani and Smyth, 2002). In this study, we detected extensive infiltration of CD4+ and CD8+ T cells in the bursa of IBDV-infected chickens. We demonstrated for the first time, the upregulated gene expression of cytolytic molecules PFN, Gzm-A, DNA repair and apoptosis molecules, HMG and PAPR in bursal cells of IBDV-infected chickens. Most importantly, the infiltrating CD4+ and CD8+ T cells were activated as evidenced by PFN production and IFN-γ expression in the bursal cells. Following infection, IBDV replicates extensively in B cells and causes bursal atrophy. Virus replication is accompanied with accumulation of T cells in the BF at the site of virus replication. These T cells are hypothesized to mediate the virus clearance in 7-10 days after infection. As demonstrated previously (Eldaghayes et al., 2006; Kim et al., 2000; Tanimura and Sharma, 1997), we also noted extensive virus replication and CD4+ and CD8+ T cell accumulation in the bursa during the acute phase of infection at PIDs 3, 5 and 7. Consistent with the previous findings (Kim et al., 2000), we also observed peak CD8+ T cell infiltration at PID 7. Similar to our findings, acute HIV infection also induced CD4+ and CD8+ T cells infiltration in the gastric mucosa of HIV-infected humans (Epple et al., 2010). 110 To understand the mechanisms underlying T cell mediated virus clearance from the bursa, we examined the expression of PFN and Gzm-A. Cytotoxic T cells kill virusinfected cells through the release of lytic proteins mainly PFN and Gzms that are secreted via exocytosis of pre-formed granules following recognition of infected targets (Hersperger et al., 2010; Kuerten et al., 2008). We detected higher expression of cytolytic proteins Gzm-A and PFN in bursal cells and PFN producing CD4+ and CD8+ T cells indicating the involvement of PFN and Gzms mediated cytotoxic mechanisms for the clearance of IBDV. In several human viral infections, CTLs play a crucial role in clearance of the virus during the acute phase of infection. For example, in poliovirus infection, cytotoxic T cells are activated, secrete IFN-γ in response to poliovirus antigen and are cytotoxic via PFN and Gzm pathway (Wahid et al., 2005). Similarly, CTLs clear the herpes simplex virus infection via PFN mediated cytotoxic mechanisms (Dobbs et al., 2005). Although we observed the gene expression of Gzm-A, we were not able to detect Gzm-A in IBDV- infected bursal tissues. This is most likely due to the lack of cross reactivity of polyclonal anti-human Gzm-A antibody with chicken. The expression of PFN was detected at PID 3 and 5 but was downregulated at PID 7 whereas Gzm-A expression was upregulated at all the PIDs tested. This differential expression of PFN and Gzm-A may be related to different stages of CTL activation. PFN expression has been shown to precede functional cytotoxicity by 12 to 24 hours (Lichtenheld and Podack, 1989). In addition, PFN may induce a cell redistribution of Gzm-A preceding apoptosis. PFN is localized together with Gzm-A in cytolytic granules of CD8+ T cells. In mammals, a study of PFN and Gzm promotor sequences has indicated 111 that these proteins may be differentially regulated at a transcriptional level (Lichtenheld and Podack, 1989). Signal requirements for PFN and Gzm-A mRNA induction may be different (Salcedo et al., 1993). We detected strong expression of HMG in bursal cells at PID 3, 5 and 7. Expression of HMG has been detected in lymphoid tissues of chickens (Pedrini et al., 1992). As HMG is cleaved during activation of the SET complex by Gzm-A, higher expression of HMG was expected. Higher expression of HMG was also detected during the cytolytic phases of MDV infection in chickens (Sarson et al., 2008). Similarly, higher expression PARP was also observed in the virus-infected bursa. In the exocytosis pathway mediated by Gzm-A, PARP activation results in depletion of cellular energy reserves thereby facilitating the cell apoptosis (Pinkoski and Green, 2003). PARP protein involvement in virus-induced apoptosis has been demonstrated in several viral infections (Mok et al., 2007; Nargi-Aizenman et al., 2002). Typically, CD4+ T cells are better known to provide helper functions for antigen presenting cells and can restrict viral replication by secreting cytokines (Christensen et al., 1999). However, there is also data that virus-specific CD4+ T cells can directly kill infected cells (Heller et al., 2006; Misko et al., 1984). In the present study, we also detected PFN positive CD4+ T cells in IBDV-infected bursa at all PIDs tested. In humans, CD4+ T cells positive for the cytotoxic effector molecules PFN and Gzm-A were detected in peripheral blood (Appay et al., 2002). These CD4+ cytotoxic cells were fully differentiated effector cells and some were specific for the β-herpesvirus human cytomegalovirus. Similarly, in HIV-infected patients PFN positive virus-specific CD4+ 112 cytotoxic T cells were detected during the early stage of infection (Appay et al., 2002). Whether infiltrating PFN expressing CD4+ T cells have IBDV specific cytolytic function is not known. Surprisingly, in the present study, we observed downregulation of gene expression of NK lysin, which is produced by both CTLs and NK cells. The role of NK cells in the pathogenesis of IBDV is not clearly defined. In earlier studies, Kumar et al., (Kumar et al., 1998) observed severe functional impairment of NK cells isolated from IBDVinfected chickens whereas Sharma and Lee (Sharma and Lee, 1983) reported no adverse effect of IBDV infection on the NK cell activity. As we detected the PFN producing T cells in infected bursa confirming that functional T cells are present in the virus-infected bursa, we speculate that downregulated NK lysin is attributed to NK cells which might not be involved in mediating the cytotoxic response against IBDV. IFN-γ, a type II interferon, is mainly produced by activated T cells and NK cells and IFNγ secretion is widely used for the assessment of antigen specific Th1 immune responses (Meier et al., 2003; Yuan et al., 2008). IFN-γ secretion by activated T-helper cells and CTLs is suggestive of effective antiviral immunity. We also detected higher levels of IFN-γ and IL-2 in the bursa of IBDV-infected chickens, suggesting that the possible source of these Th-1 cytokines could be from activated CD4+ and CD8+ T cells. IFN-γ production enhanced the cytotoxic activities of viral antigen specific CD8+ T cells in influenza virus-infected mice and vaccine induced host responses against very virulent MDV infection in chickens (Haq et al., 2010; Ishikawa et al., 2010). 113 In this study, we demonstrated the gene expression of cytolytic molecules PFN and GzmA and IFN- γ in BF of IBDV-infected chickens. These results strongly suggest that activated cytotoxic CD4+ and CD8+ T cells mediate the clearance of IBDV infection via the PFN and Gzm-A pathway. The findings of this study will be useful for designing effective control strategies for IBDV based on vaccines that can augment T cell responses in addition to an antibody response. Further studies will be needed to specifically delineate the role of specific T cell subsets in the mediation of anti-IBDV immunity. 4.6 ACKNOWLEDGEMENTS We acknowledge the salaries and research support provided by state and federal funds appropriated to the Ohio Agriculture Research and Development Center, The Ohio State University. We are grateful to Dr. Juliette Hanson, Gregory Myers and Kingsly Berlin for their help in animal work. We acknowledge Dr. D.J. Jackwood, Dr. Anastasia Vlasova and Dr. Michele Williams for critically reviewing the manuscript. 4.7 REFERENCES Abdel-Alim, G.A., Saif, Y.M., 2001a. Detection and persistence of infectious bursal disease virus in specific-pathogen-free and commercial broiler chickens. Avian Dis. 45, 646-654. Abdel-Alim, G.A., Saif, Y.M. 2001b. Immunogenicity and antigenicity of very virulent strains of infectious bursal disease viruses. Avian Dis. 45, 92-101. Appay, V., Zaunders, J.J., Papagno, L., Sutton, J., Jaramillo, A., Waters, A., Easterbrook, P,, Grey, P,, Smith, D,, McMichael, A.J,, Cooper, D.A., Rowland-Jones, S.L,, Kelleher, A.D., 2002. Characterization of CD4(+) CTLs ex vivo. J. Immunol. 168, 5954-5958. 114 Brewer, L., LaRue, R., Hering, B., Brown, C., Njenga, M.K., 2004. Transplanting encephalomyocarditis virus-infected porcine islet cells reverses diabetes in recipient mice but also transmits the virus. Xenotransplantation. 11, 160-70. Christensen, J.P., Cardin, R.D., Branum, K.C., Doherty, P.C., 1999. CD4(+) T cellmediated control of a gamma-herpesvirus in B cell-deficient mice is mediated by IFN-gamma. Proc. Natl. Acad. Sci. U S A. 96,5135-5140. Dobbs, M.E., Strasser, J.E., Chu, C.F., Chalk, C., Milligan, G.N., 2005. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin- or Fas-mediated cytolytic mechanisms. J. Virol. 79, 14546-14554. Eldaghayes, I., Rothwell, L., Williams, A., Withers, D., Balu, S., Davison, F., Kaiser, P., 2006. Infectious bursal disease virus: strains that differ in virulence differentially modulate the innate immune response to infection in the chicken bursa. Viral Immunol. 19, 83-91. Epple, H.J., Allers, K., Troger, H., Kuhl, A., Erben, U., Fromm, M., Zeitz, M., Loddenkemper, C., Schulzke, J.D., Schneider, T., 2010. Acute HIV infection induces mucosal infiltration with CD4+ and CD8+ T cells, epithelial apoptosis, and a mucosal barrier defect. Gastroenterology. 139, 1289-1300. Giulietti, A., Overbergh, L., Valckx, D., Decallonne, B., Bouillon, R., Mathieu, C., 2001. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 25, 386-401. Haq, K., Abdul-Careem, M.F., Shanmuganthan, S., Thanthrige-Don, N., Read, L.R., Sharif, S., 2010. Vaccine-induced host responses against very virulent Marek's disease virus infection in the lungs of chickens. Vaccine. 28, 5565-5572. Heller, K.N., Gurer, C., Munz, C., 2006. Virus-specific CD4+ T cells: ready for direct attack. J. Exp. Med. 203, 805-808. Hersperger, A.R., Pereyra, F., Nason, M., Demers, K., Sheth, P., Shin, L.Y., Kovacs, C.M., Rodriguez, B., Sieg, S.F., Teixeira-Johnson, L., Gudonis, D., Goepfert, P.A., Lederman, M.M., Frank, I., Makedonas, G., Kaul, R., Walker, B.D., Betts, M.R., 2010. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 6, e1000917. Ishikawa, H., Tanaka, K., Kutsukake, E., Fukui, T., Sasaki, H., Hata, A., Noda, S,, Matsumoto, T., 2010. IFN-gamma production downstream of NKT cell activation in mice infected with influenza virus enhances the cytolytic activities of both NK cells and viral antigen-specific CD8+ T cells. Virology. 407, 325-332. Khatri, M., Palmquist, J.M., Cha, R.M., Sharma, J.M., 2005. Infection and activation of bursal macrophages by virulent infectious bursal disease virus. Virus Res. 113, 44-50. 115 Kim, I.J., You, S.K., Kim, H., Yeh, H.Y., Sharma, J.M., 2000. Characteristics of bursal T lymphocytes induced by infectious bursal disease virus. J. Virol.74, 8884-8892. Kuerten, S., Nowacki, T.M., Kleen, T.O., Asaad, R.J., Lehmann, P.V., Tary-Lehmann, M., 2008. Dissociated production of perforin, granzyme B, and IFN-gamma by HIV-specific CD8(+) cells in HIV infection. AIDS Res. Hum. Retroviruses.24, 62-71. Kumar, P.A., Das, S.K., Rao, J.R., 1998. Effect of immunostimulation on cytotoxic activity of intestinal intraepithelial lymphocytes of chickens in infectious bursal disease and Eimeria tenella infections. Acta Vet. Hung. 46, 1-11. Lichtenheld, M.G., Podack, E.R., 1989. Structure of the human perforin gene. A simple gene organization with interesting potential regulatory sequences. J. Immunol. 143,4267-4274. Misko, I.S., Pope, J.H., Hutter, R., Soszynski, T.D., Kane, R.G., 1984. HLA-DR-antigenassociated restriction of EBV-specific cytotoxic T-cell colonies. Int. J. Cancer. 33, 239-243. Mok, C.K., Lee, D.C., Cheung, C.Y., Peiris, M., Lau, A.S., 2007. Differential onset of apoptosis in influenza A virus H5N1- and H1N1-infected human blood macrophages. J. Gen. Virol. 88, 1275-1280. Mullbacher, A., Waring, P., Tha Hla R., Tran, T., Chin, S., Stehle, T., Museteanu, C., Simon, M.M., 1999. Granzymes are the essential downstream effector molecules for the control of primary virus infections by cytolytic leukocytes. Proc. Natl. Acad. Sci. U S A. 96, 13950-13955. Nagata, S., Golstein P., 1995. The Fas death factor. Science 267, 1449-1456. Nargi-Aizenman, J.L., Simbulan-Rosenthal, C.M., Kelly, T.A., Smulson, M.E., Griffin, D.E., 2002. Rapid activation of poly(ADP-ribose) polymerase contributes to Sindbis virus and staurosporine-induced apoptotic cell death. Virology. 293,164171. Palmquist, J.M., Khatri, M., Cha, R.M., Goddeeris, B.M., Walcheck, B., Sharma, J.M. 2006. In vivo activation of chicken macrophages by infectious bursal disease virus. Viral Immunol. 19, 305-315. Pedrini, M., Grunicke, H., Csordas, A., 1992. Tissue-specific pattern of nonhistone high mobility group proteins in various organs of the chicken. Electrophoresis. 13, 397-399. Pertile, T.L., Sharma, J.M., Walser, M.M., 1995. Reovirus infection in chickens primes splenic adherent macrophages to produce nitric oxide in response to T cellproduced factors. Cell Immunol. 164,207-216. 116 Peters, M.A., Lin, T.L., Wu, C.C., 2005. Real-time RT-PCR differentiation and quantitation of infectious bursal disease virus strains using dual-labeled fluorescent probes. J. Virol. Methods. 127, 87-95. Pinkoski, M.J., Green, D.R., 2003. Granzyme A: the road less traveled. Nat. Immunol. 4,106-108. Rautenschlein, S., Haase, C., 2005. Differences in the immunopathogenesis of infectious bursal disease virus (IBDV) following in ovo and post-hatch vaccination of chickens. Vet. Immunol. Immunopathol.106, 139-150. Rautenschlein, S., Yeh, H.Y., Sharma, J.M., 2002. The role of T cells in protection by an inactivated infectious bursal disease virus vaccine. Vet. Immunol. Immunopathol. 89, 159-167. Salcedo, T.W., Azzoni, L., Wolf, S.F., Perussia, B., 1993. Modulation of perforin and granzyme messenger RNA expression in human natural killer cells. J. Immunol. 151, 2511-2520. Sarin, A., Williams, M.S., Alexander-Miller, M.A., Berzofsky, J.A., Zacharchuk, C.M., Henkart, P.A., 1997. Target cell lysis by CTL granule exocytosis is independent of ICE/Ced-3 family proteases. Immunity. 6, 209-215. Sarson, A.J., Abdul-Careem, M.F., Read, L.R., Brisbin, J.T., Sharif, S., 2008. Expression of cytotoxicity-associated genes in Marek's disease virus-infected chickens. Viral Immunol. 21, 267-272. 117 Fig.4.1: Detection of virus load and IBDV genome in bursa tissue. At PIDs 3, 5 and 7, bursal tissues were collected from IBDV-infected and virus free control chickens for the quantification of virus load by qRT-PCR (A) and viral genome detection by RT-PCR (B). (A) Represents the log10 virus titer at PIDs 3, 5 and 7 in IBDV-infected chickens. Values represent the mean virus titer ±SEM of 3 chickens at each time point. (B) IBDV genome was detected in the bursa of virus-infected chickens at PIDs 3, 5 and 7 whereas viral genome was absent in mock-inoculated controls. Lane 1, 100 base pair marker, lane 2 positive control, lanes 3, 4 and 5 bursa samples from IBDV-infected chickens at PIDs 3, 5 and 7; and lanes 6, 7 and 8, BF samples from virus free control at PIDs 3, 5 and 7 respectively. 118 Figure 4.1. Detection of virus load and IBDV genome in bursa tissue. 119 Fig. 4.2: Gene expression of PFN, Gzm-A and molecules involved in DNA repair and apoptosis in the bursa of IBDV-infected chickens. At PID 3, 5 and 7, bursal mononuclear cells were isolated from IBDV-infected and virus-free control chickens and examined for the expression of PFN (A), Gzm-A (B), HMG (C), PARP (D) and NK lysin (E) by qRTPCR. The data is expressed as fold change in gene expression in infected chickens over virus free control. The values represent the mean ± SEM of 4 pools of 3 BF at each PID. 120 Figure 4.2. Gene expression of PFN, Gzm-A and molecules involved in DNA repair and apoptosis in the bursa of IBDV-infected chickens. 121 Fig. 4.3: Accumulation of CD8+ T cells in the bursa. Three-weeks-old chickens were inoculated with IBDV and frozen sections of bursa were prepared. At PIDs 3, 5 and 7 bursal sections from virus free chickens (a, b, and c) and IBDV-infected chickens (d, e, and f) were examined for the presence of CD8+ T cells by immunohistochemistry using anti-chicken CD8 mAb. Brown color shown by arrow indicates the positive staining. (B) Quantification of CD8+ T cells in the bursa. CD8+ T were counted at magnification (20X) at PID 3, 5 and 7. The values represent the mean ±SEM of 5 fields/BF/bird at each PID. *Significantly different from the control group P<0.05. 122 Figure 4.3. Accumulation of CD8+ T cells in the bursa. 123 Fig.4.4: Infiltration of CD4+ T cells in the bursa. Three-weeks-old chickens were inoculated with IBDV and frozen sections of bursa were prepared. (A) At PIDs 3, 5 and 7 bursal sections from virus free chickens (a, b, and c) and IBDV-infected chickens (d, e, and f) were examined for the presence of CD4+ T cells by immunohistochemistry using anti-chicken CD4 mAb. Brown color shown by arrow indicates the positive staining. (B) Quantification of CD4+ T cells in the bursa. CD4+ T cells were counted (20X) at PID 3, 5 and 7. The values represent the mean ± SEM of 5 fields/BF/bird at each PID. *Significantly different from the control group P<0.05. 124 Figure 4.4. Infiltration of CD4+ T cells in the bursa. 125 Fig. 4.5: Detection of PFN producing CD4+ and CD8+ T cells in BF. (A) Chickens were inoculated with IBDV and bursal tissues were collected. At 3, 5 and 7 PID bursal sections from virus free chickens (a, b and c) and IBDV-infected chickens (d, e, and f) were examined (40X) for the presence of PFN by immunohistochemistry using anti-human PFN mAb. Development of brown color shown by arrow indicates positive staining for PFN. (B) Double staining for CD4+ and (C) for CD8+cells, positive for PFN; blue staining shown by arrow head represents CD4+ or CD8+ positive T cells and brown staining shows PFN positive cells respectively (D) The numbers of PFN positive cells were counted (20X) at PID 3, 5 and 7. The values represent mean ± SEM of 5 fields/BF/bird at each PID. *Significantly different from the control group P<0.05. 126 Figure 4.5. Detection of PFN producing CD4+ and CD8+ T cells in BF. 127 Fig. 4.6: Expression of Th-1 cytokines in the bursa of IBDV-infected chickens at PIDs 3, 5 and 7. (A) IL-2 and (B) IFN-γ gene expression was quantified in bursal mononuclear cells. The data is expressed as fold change in gene expression in infected chickens over virus free control. The values represent the mean ±SEM of 4 pools of 3 BF at each PID. 128 Figure 4.6. Expression of Th-1 cytokines in the bursa of IBDV-infected chickens at PIDs 3, 5 and 7. 129 BIBLIOGRAPHY Abdel-Alim, G.A. and Saif, Y.M. (2001a) Detection and persistence of infectious bursal disease virus in specific-pathogen-free and commercial broiler chickens. Avian Dis 45(3), 646-54. Abdel-Alim, G.A. and Saif, Y.M. (2001b) Immunogenicity and antigenicity of very virulent strains of infectious bursal disease viruses. Avian Dis 45(1), 92-101. Al-Natour, M.Q., Ward, L.A., Saif, Y.M., Stewart-Brown, B. and Keck, L.D. (2004) Effect of diffferent levels of maternally derived antibodies on protection against infectious bursal disease virus. Avian Dis 48(1), 177-82. Allan, W.H., Faragher, J.T. and Cullen, G.A. (1972) Immunosuppression by the infectious bursal agent in chickens immunised against Newcastle disease. Vet Rec 90(18), 511-2. Anderson, W.I., Reid, W.M., Lukert, P.D. and Fletcher, O.J., Jr. (1977) Influence of infectious bursal disease on the development of immunity to Eimeria tenella. Avian Dis 21(4), 637-41. Andrejeva, J., Childs, K.S., Young, D.F., Carlos, T.S., Stock, N., Goodbourn, S. and Randall, R.E. (2004) The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci U S A 101(49), 17264-9. Anon. (1999) International Animal Health Code: mammals, birds and bees. Office International des Epizooties; 8th ed. Office International des Epizooties 8th ed. Appay, V., Zaunders, J.J., Papagno, L., Sutton, J., Jaramillo, A., Waters, A., Easterbrook, P., Grey, P., Smith, D., McMichael, A.J., Cooper, D.A., Rowland-Jones, S.L. and Kelleher, A.D. (2002) Characterization of CD4(+) CTLs ex vivo. J Immunol 168(11), 5954-8. Ashraf, S., Abdel-Alim, G. and Saif, Y.M. (2006) Detection of antibodies against serotypes 1 and 2 infectious bursal disease virus by commercial ELISA kits. Avian Dis 50(1), 104-9. 130 Abdel-Alim, G.A. and Saif, Y.M. (2001a) Detection and persistence of infectious bursal disease virus in specific-pathogen-free and commercial broiler chickens. Avian Dis 45(3), 646-54. Abdel-Alim, G.A. and Saif, Y.M. (2001b) Immunogenicity and antigenicity of very virulent strains of infectious bursal disease viruses. Avian Dis 45(1), 92-101. Abdul-Careem, M.F., Haq, K., Shanmuganathan, S., Read, L.R., Schat, K.A., Heidari, M. and Sharif, S. (2009) Induction of innate host responses in the lungs of chickens following infection with a very virulent strain of Marek's disease virus. Virology 393(2), 250-7. Al-Natour, M.Q., Ward, L.A., Saif, Y.M., Stewart-Brown, B. and Keck, L.D. (2004) Effect of diffferent levels of maternally derived antibodies on protection against infectious bursal disease virus. Avian Dis 48(1), 177-82. Allan, W.H., Faragher, J.T. and Cullen, G.A. (1972) Immunosuppression by the infectious bursal agent in chickens immunised against Newcastle disease. Vet Rec 90(18), 511-2. Anderson, W.I., Reid, W.M., Lukert, P.D. and Fletcher, O.J., Jr. (1977) Influence of infectious bursal disease on the development of immunity to Eimeria tenella. Avian Dis 21(4), 637-41. Andrejeva, J., Childs, K.S., Young, D.F., Carlos, T.S., Stock, N., Goodbourn, S. and Randall, R.E. (2004) The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci U S A 101(49), 17264-9. Appay, V., Zaunders, J.J., Papagno, L., Sutton, J., Jaramillo, A., Waters, A., Easterbrook, P., Grey, P., Smith, D., McMichael, A.J., Cooper, D.A., Rowland-Jones, S.L. and Kelleher, A.D. (2002) Characterization of CD4(+) CTLs ex vivo. J Immunol 168(11), 5954-8. Ashraf, S., Abdel-Alim, G. and Saif, Y.M. (2006) Detection of antibodies against serotypes 1 and 2 infectious bursal disease virus by commercial ELISA kits. Avian Dis 50(1), 104-9. Australia. (2001 ) Generic import risk analysis (IRA) for uncooked chicken meat. IRA Report, 35. Azad, A.A., Jagadish, M.N., Brown, M.A. and Hudson, P.J. (1987) Deletion mapping and expression in Escherichia coli of the large genomic segment of a birnavirus. Virology 161(1), 145-52. 131 Bacon, L.D., Fadly, A.M. and Crittenden, L.B. (1986) Absence of influence on immune competence by the sex-linked gene (K) determining slow feathering in white leghorn chickens. Avian Dis 30(4), 751-60. Barker, K.A., Hampe, A., Stoeckle, M.Y. and Hanafusa, H. (1993) Transformationassociated cytokine 9E3/CEF4 is chemotactic for chicken peripheral blood mononuclear cells. J Virol 67(6), 3528-33. Bautista, D.A., Elankumaran, S. and Heckert, R.A. (2004) Effect of a variant infectious bursal disease virus (E/Del) on Salmonella typhimurium infection in commercial broiler chickens. Avian Dis 48(2), 361-9. Becht, H. (1980) Infectious bursal disease virus. Curr Top Microbiol Immunol 90, 10721. Becht, H. and Muller, H. (1991) Infectious bursal disease--B cell dependent immunodeficiency syndrome in chickens. Behring Inst Mitt(89), 217-25. Berg, T.P. and Meulemans, G. (1991) Acute infectious bursal disease in poultry: protection afforded by maternally derived antibodies and interference with live vaccination. Avian Pathol 20(3), 409-21. Brewer, L., LaRue, R., Hering, B., Brown, C. and Njenga, M.K. (2004) Transplanting encephalomyocarditis virus-infected porcine islet cells reverses diabetes in recipient mice but also transmits the virus. Xenotransplantation 11(2), 160-70. Burkhardt, E. and Muller, H. (1987) Susceptibility of chicken blood lymphoblasts and monocytes to infectious bursal disease virus (IBDV). Arch Virol 94(3-4), 297303. Chettle, N., Stuart, J.C. and Wyeth, P.J. (1989) Outbreak of virulent infectious bursal disease in East Anglia. Vet Rec 125(10), 271-2. Christensen, J.P., Cardin, R.D., Branum, K.C. and Doherty, P.C. (1999) CD4(+) T cellmediated control of a gamma-herpesvirus in B cell-deficient mice is mediated by IFN-gamma. Proc Natl Acad Sci U S A 96(9), 5135-40. Confer, A.W. and MacWilliams, P.S. (1982) Correlation of hematological changes and serum and monocyte inhibition with the early suppression of phytohemagglutinin stimulation of lymphocytes in experimental infectious bursal disease. Can J Comp Med 46(2), 169-75. Confer, A.W., Springer, W.T., Shane, S.M. and Donovan, J.F. (1981) Sequential mitogen stimulation of peripheral blood lymphocytes from chickens inoculated with infectious bursal disease virus. Am J Vet Res 42(12), 2109-13. 132 Craft, D.W., Brown, J. and Lukert, P.D. (1990) Effects of standard and variant strains of infectious bursal disease virus on infections of chickens. Am J Vet Res 51(8), 1192-7. Delgui, L., Gonzalez, D. and Rodriguez, J.F. (2009) Infectious bursal disease virus persistently infects bursal B-lymphoid DT40 cells. J Gen Virol 90(Pt 5), 1148-52. Dobbs, M.E., Strasser, J.E., Chu, C.F., Chalk, C. and Milligan, G.N. (2005) Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin- or Fas-mediated cytolytic mechanisms. J Virol 79(23), 14546-54. Dohms, J.E. and Jaeger, J.S. (1988) The effect of infectious bursal disease virus infection on local and systemic antibody responses following infection of 3-week-old broiler chickens. Avian Dis 32(4), 632-40. Dohms, J.E., Lee, K.P. and Rosenberger, J.K. (1981) Plasma cell changes in the gland of Harder following infectious bursal disease virus infection of the chicken. Avian Dis 25(3), 683-95. Elankumaran, S., Heckert, R.A. and Moura, L. (2002) Pathogenesis and tissue distribution of a variant strain of infectious bursal disease virus in commercial broiler chickens. Avian Dis 46(1), 169-76. Eldaghayes, I., Rothwell, L., Williams, A., Withers, D., Balu, S., Davison, F. and Kaiser, P. (2006) Infectious bursal disease virus: strains that differ in virulence differentially modulate the innate immune response to infection in the chicken bursa. Viral Immunol 19(1), 83-91. Epple, H.J., Allers, K., Troger, H., Kuhl, A., Erben, U., Fromm, M., Zeitz, M., Loddenkemper, C., Schulzke, J.D. and Schneider, T. (2010) Acute HIV infection induces mucosal infiltration with CD4+ and CD8+ T cells, epithelial apoptosis, and a mucosal barrier defect. Gastroenterology 139(4), 1289-300. Fahey, K.J., O'Donnell, I.J. and Bagust, T.J. (1985) Antibody to the 32K structural protein of infectious bursal disease virus neutralizes viral infectivity in vitro and confers protection on young chickens. J Gen Virol 66 ( Pt 12), 2693-702. Faragher, J.T., Allan, W.H. and Cullen, G.A. (1972) Immunosuppressive effect of the infectious bursal agent in the chicken. Nat New Biol 237(73), 118-9. Fernandez-Arias, A., Martinez, S. and Rodriguez, J.F. (1997) The major antigenic protein of infectious bursal disease virus, VP2, is an apoptotic inducer. J Virol 71(10), 8014-8. Flensburg, M.F., Ersboll, A.K. and Jorgensen, P.H. (2002) Risk factors associated with the introduction of acute clinical infectious bursal disease among Danish broiler chickens in 1998. Avian Pathol 31(1), 23-9. 133 Giambrone, J.J., Anderson, W.I., Reid, W.M. and Eidson, C.S. (1977a) Effect of infectious bursal disease on the severity of Eimeria tenella infections in broiler chicks. Poult Sci 56(1), 243-6. Giambrone, J.J. and Clay, R.P. (1986) Evaluation of the immunogenicity, stability, pathogenicity, and immunodepressive potential of four commercial live infectious bursal disease vaccines. Poult Sci 65(7), 1287-90. Giambrone, J.J., Eidson, C.S. and Kleven, S.H. (1977b) Effect of infectious bursal disease on the response of chickens to Mycoplasma synoviae, Newcastle disease virus, and infectious bronchitis virus. Am J Vet Res 38(2), 251-3. Giulietti, A., Overbergh, L., Valckx, D., Decallonne, B., Bouillon, R. and Mathieu, C. (2001) An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25(4), 386-401. Glass, W.G., Rosenberg, H.F. and Murphy, P.M. (2003) Chemokine regulation of inflammation during acute viral infection. Curr Opin Allergy Clin Immunol 3(6), 467-73. Granzow, H., Birghan, C., Mettenleiter, T.C., Beyer, J., Kollner, B. and Mundt, E. (1997) A second form of infectious bursal disease virus-associated tubule contains VP4. J Virol 71(11), 8879-85. Haq, K., Abdul-Careem, M.F., Shanmuganthan, S., Thanthrige-Don, N., Read, L.R. and Sharif, S. (2010) Vaccine-induced host responses against very virulent Marek's disease virus infection in the lungs of chickens. Vaccine 28(34), 5565-72. Hassan, M.K., Al-Natour, M.Q., Ward, L.A. and Saif, Y.M. (1996) Pathogenicity, attenuation, and immunogenicity of infectious bursal disease virus. Avian Dis 40(3), 567-71. Hassan, M.K. and Saif, Y.M. (1996) Influence of the host system on the pathogenicity, immunogenicity, and antigenicity of infectious bursal disease virus. Avian Dis 40(3), 553-61. Heil, F., Hemmi, H., Hochrein, H., Ampenberger, F., Kirschning, C., Akira, S., Lipford, G., Wagner, H. and Bauer, S. (2004) Species-specific recognition of singlestranded RNA via toll-like receptor 7 and 8. Science 303(5663), 1526-9. Heitmeier, M.R., Scarim, A.L. and Corbett, J.A. (1998) Double-stranded RNA-induced inducible nitric-oxide synthase expression and interleukin-1 release by murine macrophages requires NF-kappaB activation. J Biol Chem 273(24), 15301-7. Heller, K.N., Gurer, C. and Munz, C. (2006) Virus-specific CD4+ T cells: ready for direct attack. J Exp Med 203(4), 805-8. 134 Henderson, K.S. and Jackwood, D.J. (1990) Comparison of the dot blot hybridization assay with antigen detection assays for the diagnosis of infectious bursal disease virus infections. Avian Dis 34(3), 744-8. Hersperger, A.R., Pereyra, F., Nason, M., Demers, K., Sheth, P., Shin, L.Y., Kovacs, C.M., Rodriguez, B., Sieg, S.F., Teixeira-Johnson, L., Gudonis, D., Goepfert, P.A., Lederman, M.M., Frank, I., Makedonas, G., Kaul, R., Walker, B.D. and Betts, M.R. (2010) Perforin expression directly ex vivo by HIV-specific CD8 Tcells is a correlate of HIV elite control. PLoS Pathog 6(5), e1000917. Hghihghi, H.R., Read, L.R., Mohammadi, H., Pei, Y., Ursprung, C., Nagy, E., Behboudi, S., Haeryfar, S.M. and Sharif, S. (2010) Characterization of host responses against a recombinant fowlpox virus-vectored vaccine expressing the hemagglutinin antigen of an avian influenza virus. Clin Vaccine Immunol 17(3), 454-63. Higashihara, M., Saijo, K., Fujisaki, Y. and Matumoto, M. (1991) Immunosuppressive effect of infectious bursal disease virus strains of variable virulence for chickens. Vet Microbiol 26(3), 241-8. Hirai, K., Funakoshi, T., Nakai, T. and Shimakura, S. (1981) Sequential changes in the number of surface immunoglobulin-bearing B lymphocytes in infectious bursal disease virus-infected chickens. Avian Dis 25(2), 484-96. Hirai, K., Kunihiro, K. and Shimakura, S. (1979) Characterization of Immunosuppression in chickens by infectious bursal disease virus. Avian Dis 23(4), 950-65. Hirai, K., Shimakura, S., Kawamoto, E., Taguchi, F., Kim, S.T., Chang, C.N. and Iritani, Y. (1974) The immunodepressive effect of infectious bursal disease virus in chickens. Avian Dis 18(1), 50-7. Hudson, L., Pattison, M. and Thantrey, N. (1975) Specific B lymphocyte suppression by infectious bursal agent (Gumboro disease virus) in chickens. Eur J Immunol 5(10), 675-9. Inoue, M., Fukuda, M. and Miyano, K. (1994) Thymic lesions in chicken infected with infectious bursal disease virus. Avian Dis 38(4), 839-46. Inoue, M., Satoh, W. and Murakami, H. (1997) Plasma alpha 1-acid glycoprotein in chickens infected with infectious bursal disease virus. Avian Dis 41(1), 164-70. Ishikawa, H., Tanaka, K., Kutsukake, E., Fukui, T., Sasaki, H., Hata, A., Noda, S. and Matsumoto, T. (2010) IFN-gamma production downstream of NKT cell activation in mice infected with influenza virus enhances the cytolytic activities of both NK cells and viral antigen-specific CD8+ T cells. Virology 407(2), 325-32. Ismail, N.M., Fadly, A.M. and Chang, T.S. (1987) Effect of bursal cell number on the pathogenesis of infectious bursal disease in chickens. Avian Dis 31(3), 546-55. 135 Ismail, N.M. and Saif, Y.M. (1991) Immunogenicity of infectious bursal disease viruses in chickens. Avian Dis 35(3), 460-9. Ismail, N.M., Saif, Y.M., Wigle, W.L., Havenstein, G.B. and Jackson, C. (1990) Infectious bursal disease virus variant from commercial Leghorn pullets. Avian Dis 34(1), 141-5. Ivanyi, J. (1975) Immunodeficiency in the chicken. II. Production of monomeric IgM following testosterone treatment or infection with Gumboro disease. Immunology 28(6), 1015-21. Ivanyi, J. and Morris, R. (1976) Immunodeficiency in the chicken. IV. An immunological study of infectious bursal disease. Clin Exp Immunol 23(1), 154-65. Jackwood, D.H. and Saif, Y.M. (1987) Antigenic diversity of infectious bursal disease viruses. Avian Dis 31(4), 766-70. Jackwood, D.J., Cookson, K.C., Sommer-Wagner, S.E., Le Galludec, H. and de Wit, J.J. (2006) Molecular characteristics of infectious bursal disease viruses from asymptomatic broiler flocks in Europe. Avian Dis 50(4), 532-6. Jackwood, D.J., Saif, Y.M. and Moorhead, P.D. (1985) Immunogenicity and antigenicity of infectious bursal disease virus serotypes I and II in chickens. Avian Dis 29(4), 1184-94. Jackwood, D.J. and Sommer-Wagner, S.E. (2005) Molecular epidemiology of infectious bursal disease viruses: distribution and genetic analysis of newly emerging viruses in the United States. Avian Dis 49(2), 220-6. Jackwood, D.J. and Sommer-Wagner, S.E. (2010) Detection and characterization of infectious bursal disease viruses in broilers at processing. Prev Vet Med 97(1), 45-50. Jackwood, D.J., Sommer-Wagner, S.E., Stoute, A.S., Woolcock, P.R., Crossley, B.M., Hietala, S.K. and Charlton, B.R. (2009) Characteristics of a very virulent infectious bursal disease virus from California. Avian Dis 53(4), 592-600. Jarosinski, K.W., Yunis, R., O'Connell, P.H., Markowski-Grimsrud, C.J. and Schat, K.A. (2002) Influence of genetic resistance of the chicken and virulence of Marek's disease virus (MDV) on nitric oxide responses after MDV infection. Avian Dis 46(3), 636-49. Jiang, Z., Mak, T.W., Sen, G. and Li, X. (2004) Toll-like receptor 3-mediated activation of NF-kappaB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-beta. Proc Natl Acad Sci U S A 101(10), 3533-8. 136 Jungmann, A., Nieper, H. and Muller, H. (2001) Apoptosis is induced by infectious bursal disease virus replication in productively infected cells as well as in antigennegative cells in their vicinity. J Gen Virol 82(Pt 5), 1107-15. Karpala, A.J., Lowenthal, J.W. and Bean, A.G. (2008) Activation of the TLR3 pathway regulates IFNbeta production in chickens. Dev Comp Immunol 32(4), 435-44. Kaufer, I. and Weiss, E. (1976) Electron-microscope studies on the pathogenesis of infectious bursal disease after intrabursal application of the causal virus. Avian Dis 20(3), 483-95. Kaufer, I. and Weiss, E. (1980) Significance of bursa of Fabricius as target organ in infectious bursal disease of chickens. Infect Immun 27(2), 364-7. Kawai, T. and Akira, S. (2006) Innate immune recognition of viral infection. Nat Immunol 7(2), 131-7. Kawai, T. and Akira, S. (2009) The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol 21(4), 317-37. Khatri, M., Palmquist, J.M., Cha, R.M. and Sharma, J.M. (2005) Infection and activation of bursal macrophages by virulent infectious bursal disease virus. Virus Res 113(1), 44-50. Khatri, M. and Sharma, J.M. (2006) Infectious bursal disease virus infection induces macrophage activation via p38 MAPK and NF-kappaB pathways. Virus Res 118(1-2), 70-7. Kim, I.J., Gagic, M. and Sharma, J.M. (1999) Recovery of antibody-producing ability and lymphocyte repopulation of bursal follicles in chickens exposed to infectious bursal disease virus. Avian Dis 43(3), 401-13. Kim, I.J., Karaca, K., Pertile, T.L., Erickson, S.A. and Sharma, J.M. (1998) Enhanced expression of cytokine genes in spleen macrophages during acute infection with infectious bursal disease virus in chickens. Vet Immunol Immunopathol 61(2-4), 331-41. Kim, I.J. and Sharma, J.M. (2000) IBDV-induced bursal T lymphocytes inhibit mitogenic response of normal splenocytes. Vet Immunol Immunopathol 74(1-2), 47-57. Kim, I.J., You, S.K., Kim, H., Yeh, H.Y. and Sharma, J.M. (2000) Characteristics of bursal T lymphocytes induced by infectious bursal disease virus. J Virol 74(19), 8884-92. Komine, K., Ohta, H., Kamata, S., Uchida, K., Yoshikawa, Y., Yamanouchi, K. and Okada, H. (1989) Complement activation by infectious bursal disease virus and 137 its relevance to virus growth in the lymphoid cells. Nippon Juigaku Zasshi 51(6), 1259-62. Kuerten, S., Nowacki, T.M., Kleen, T.O., Asaad, R.J., Lehmann, P.V. and TaryLehmann, M. (2008) Dissociated production of perforin, granzyme B, and IFNgamma by HIV-specific CD8(+) cells in HIV infection. AIDS Res Hum Retroviruses 24(1), 62-71. Kumar, P.A., Das, S.K. and Rao, J.R. (1998) Effect of immunostimulation on cytotoxic activity of intestinal intraepithelial lymphocytes of chickens in infectious bursal disease and Eimeria tenella infections. Acta Vet Hung 46(1), 1-11. Lam, K.M. (1991) Infectious bursal disease virus type 1-induced suppression of chicken lymphocyte response to mitogen. Avian Pathol 20(2), 205-12. Lam, K.M. (1997) Morphological evidence of apoptosis in chickens infected with infectious bursal disease virus. J Comp Pathol 116(4), 367-77. Lange, H., Muller, H., Kaufer, I. and Becht, H. (1987) Pathogenic and structural properties of wild type infectious bursal disease virus (IBDV) and virus grown in vitro. Arch Virol 92(3-4), 187-96. Lasher, H.N. and Davis, V.S. (1997) History of infectious bursal disease in the U.S.A.-the first two decades. Avian Dis 41(1), 11-9. Li, G., Lillehoj, H.S., Lee, K.W., Jang, S.I., Marc, P., Gay, C.G., Ritter, G.D., Bautista, D.A., Phillips, K., Neumann, A.P., Rehberger, T.G. and Siragusa, G.R. (2010) An outbreak of gangrenous dermatitis in commercial broiler chickens. Avian Pathol 39(4), 247-53. Lichtenheld, M.G. and Podack, E.R. (1989) Structure of the human perforin gene. A simple gene organization with interesting potential regulatory sequences. J Immunol 143(12), 4267-74. Lim, B.L., Cao, Y., Yu, T. and Mo, C.W. (1999) Adaptation of very virulent infectious bursal disease virus to chicken embryonic fibroblasts by site-directed mutagenesis of residues 279 and 284 of viral coat protein VP2. J Virol 73(4), 2854-62. Lukert P.D., S., Y.M.,, ed. (2003) Infectious bursal disease. 11th ed. Diseases of Poultry. Edited by B. Saif Y.M., H.J., Glisson, J.R., Fadly, A.M., McDougald, L.R., Swayne, D.E. . Iowa State University Press, Ames. Luque, D., Rivas, G., Alfonso, C., Carrascosa, J.L., Rodriguez, J.F. and Caston, J.R. (2009) Infectious bursal disease virus is an icosahedral polyploid dsRNA virus. Proc Natl Acad Sci U S A 106(7), 2148-52. 138 Mackenzie, M. and Spradbrow, P.B. (1981) Persistence of infectious bursal disease virus in experimentally infected chickens. Aust Vet J 57(11), 534-5. Mandeville, W.F., Cook, F.K. and Jackwood, D.J. (2000) Heat lability of five strains of infectious bursal disease virus. Poult Sci 79(6), 838-42. Mazariegos, L.A., Lukert, P.D. and Brown, J. (1990) Pathogenicity and immunosuppressive properties of infectious bursal disease "intermediate" strains. Avian Dis 34(1), 203-8. McFerran, J.B., M. S. Mcnulty, F. R. Mckillop, T. J. Connor, R. M. Mccracken, D. S. Collins, and G. M. Allan. . (1980) Isolation and serological studies with infectious bursal disease viruses from fowl, turkeys and ducks— demonstration of a 2nd serotype. . Avian Pathol 9, 395–404. Meier, W.A., Galeota, J., Osorio, F.A., Husmann, R.J., Schnitzlein, W.M. and Zuckermann, F.A. (2003) Gradual development of the interferon-gamma response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology 309(1), 18-31. Misko, I.S., Pope, J.H., Hutter, R., Soszynski, T.D. and Kane, R.G. (1984) HLA-DRantigen-associated restriction of EBV-specific cytotoxic T-cell colonies. Int J Cancer 33(2), 239-43. Mogensen, T.H., Melchjorsen, J., Malmgaard, L., Casola, A. and Paludan, S.R. (2004) Suppression of proinflammatory cytokine expression by herpes simplex virus type 1. J Virol 78(11), 5883-90. Mok, C.K., Lee, D.C., Cheung, C.Y., Peiris, M. and Lau, A.S. (2007) Differential onset of apoptosis in influenza A virus H5N1- and H1N1-infected human blood macrophages. J Gen Virol 88(Pt 4), 1275-80. Montgomery, R.D. and Maslin, W.R. (1991) Effect of infectious bursal disease virus vaccines on persistence and pathogenicity of modified live reovirus vaccines in chickens. Avian Dis 35(1), 147-57. Mullbacher, A., Waring, P., Tha Hla, R., Tran, T., Chin, S., Stehle, T., Museteanu, C. and Simon, M.M. (1999) Granzymes are the essential downstream effector molecules for the control of primary virus infections by cytolytic leukocytes. Proc Natl Acad Sci U S A 96(24), 13950-5. Muller, H. (1986) Replication of infectious bursal disease virus in lymphoid cells. Arch Virol 87(3-4), 191-203. Muller, H., Islam, M.R. and Raue, R. (2003) Research on infectious bursal disease--the past, the present and the future. Vet Microbiol 97(1-2), 153-65. 139 Muller, R., Kaufer, I., Reinacher, M. and Weiss, E. (1979) Immunofluorescent studies of early virus propagation after oral infection with infectious bursal disease virus (IBDV). Zentralbl Veterinarmed B 26(5), 345-52. Mundt, E., de Haas, N. and van Loon, A.A. (2003) Development of a vaccine for immunization against classical as well as variant strains of infectious bursal disease virus using reverse genetics. Vaccine 21(31), 4616-24. Nagata, S. and Golstein, P. (1995) The Fas death factor. Science 267(5203), 1449-56. Nargi-Aizenman, J.L., Simbulan-Rosenthal, C.M., Kelly, T.A., Smulson, M.E. and Griffin, D.E. (2002) Rapid activation of poly(ADP-ribose) polymerase contributes to Sindbis virus and staurosporine-induced apoptotic cell death. Virology 293(1), 164-71. Nieper, H. and Muller, H. (1996) Susceptibility of chicken lymphoid cells to infectious bursal disease virus does not correlate with the presence of specific binding sites. J Gen Virol 77 ( Pt 6), 1229-37. Ojeda, F., Skardova, I., Guarda, M.I., Ulloa, J. and Folch, H. (1997) Proliferation and apoptosis in infection with infectious bursal disease virus: a flow cytometric study. Avian Dis 41(2), 312-6. Okoye, J.O. and Shoyinka, S.V. (1983) Newcastle disease in a vaccinated flock which had experienced subclinical infectious bursal disease. Trop Anim Health Prod 15(4), 221-5. Palmquist, J.M., Khatri, M., Cha, R.M., Goddeeris, B.M., Walcheck, B. and Sharma, J.M. (2006) In vivo activation of chicken macrophages by infectious bursal disease virus. Viral Immunol 19(2), 305-15. Pedrini, M., Grunicke, H. and Csordas, A. (1992) Tissue-specific pattern of nonhistone high mobility group proteins in various organs of the chicken. Electrophoresis 13(6), 397-9. Pejkovski, C., Davelaar, F.G. and Kouwenhoven, B. (1979) Immunosuppressive effect of infectious bursal disease virus on vaccination against infectious bronchitis. Avian Pathol 8(1), 95-106. Pertile, T.L., Sharma, J.M. and Walser, M.M. (1995) Reovirus infection in chickens primes splenic adherent macrophages to produce nitric oxide in response to T cell-produced factors. Cell Immunol 164(2), 207-16. Peters, M.A., Lin, T.L. and Wu, C.C. (2005) Real-time RT-PCR differentiation and quantitation of infectious bursal disease virus strains using dual-labeled fluorescent probes. J Virol Methods 127(1), 87-95. 140 Pinkoski, M.J. and Green, D.R. (2003) Granzyme A: the road less traveled. Nat Immunol 4(2), 106-8. Poonia, B. and Charan, S. (2005) Early and transient induction of nitric oxide (NO) in infectious bursal disease virus infection is T-cell dependent: a study in cyclosporin-A treated chicken-model. Indian J Exp Biol 43(2), 192-6. Ramirez-Nieto, G., Shivaprasad, H.L., Kim, C.H., Lillehoj, H.S., Song, H., Osorio, I.G. and Perez, D.R. (2010) Adaptation of a mallard H5N2 low pathogenicity influenza virus in chickens with prior history of infection with infectious bursal disease virus. Avian Dis 54(1 Suppl), 513-21. Rauf, A., Khatri, M., Murgia, M.V. and Saif, Y.M. (2011) Expression of perforingranzyme pathway genes in the bursa of infectious bursal disease virus-infected chickens. Dev Comp Immunol. Rautenschlein, S. and Haase, C. (2005) Differences in the immunopathogenesis of infectious bursal disease virus (IBDV) following in ovo and post-hatch vaccination of chickens. Vet Immunol Immunopathol 106(1-2), 139-50. Rautenschlein, S., von Samson-Himmelstjerna, G. and Haase, C. (2007) A comparison of immune responses to infection with virulent infectious bursal disease virus (IBDV) between specific-pathogen-free chickens infected at 12 and 28 days of age. Vet Immunol Immunopathol 115(3-4), 251-60. Rautenschlein, S., Yeh, H.Y., Njenga, M.K. and Sharma, J.M. (2002a) Role of intrabursal T cells in infectious bursal disease virus (IBDV) infection: T cells promote viral clearance but delay follicular recovery. Arch Virol 147(2), 285-304. Rautenschlein, S., Yeh, H.Y. and Sharma, J.M. (2002b) The role of T cells in protection by an inactivated infectious bursal disease virus vaccine. Vet Immunol Immunopathol 89(3-4), 159-67. Rautenschlein, S., Yeh, H.Y. and Sharma, J.M. (2003) Comparative immunopathogenesis of mild, intermediate, and virulent strains of classic infectious bursal disease virus. Avian Dis 47(1), 66-78. Reed, L.J. and Muench, H.A. (1983) A simple method of estimation fifty per cent endpoint. . Am. J. Hyg 27, 493-497. Rodenberg, J., Sharma, J.M., Belzer, S.W., Nordgren, R.M. and Naqi, S. (1994) Flow cytometric analysis of B cell and T cell subpopulations in specific-pathogen-free chickens infected with infectious bursal disease virus. Avian Dis 38(1), 16-21. Rosenberger, J.K. and Gelb, J., Jr. (1978) Response to several avian respiratory viruses as affected by infectious bursal disease virus. Avian Dis 22(1), 95-105. 141 Rosenberger, J.K., Klopp, S., Eckroade, R.J. and Krauss, W.C. (1975) The roles of the infectious bursal agent and several avian adenoviruses in the hemorrhagicaplastic-anemia syndrome and gangrenous dermatitis. Avian Dis 19(4), 717-29. Saif, Y.M. (1991) Immunosuppression induced by infectious bursal disease virus. Vet Immunol Immunopathol 30(1), 45-50. Saif, Y.M. (1998) Infectious bursal disease and hemorrhagic enteritis. Poult Sci 77(8), 1186-9. Saif, Y.M. (2004) Control of infectious bursal disease virus by vaccination. Dev Biol (Basel) 119, 143-6. Salcedo, T.W., Azzoni, L., Wolf, S.F. and Perussia, B. (1993) Modulation of perforin and granzyme messenger RNA expression in human natural killer cells. J Immunol 151(5), 2511-20. Sanchez, J., Stryhn, H., Flensburg, M., Ersboll, A.K. and Dohoo, I. (2005) Temporal and spatial analysis of the 1999 outbreak of acute clinical infectious bursal disease in broiler flocks in Denmark. Prev Vet Med 71(3-4), 209-23. Sang, Y., Ross, C.R., Rowland, R.R. and Blecha, F. (2008) Toll-like receptor 3 activation decreases porcine arterivirus infection. Viral Immunol 21(3), 303-13. Sarin, A., Williams, M.S., Alexander-Miller, M.A., Berzofsky, J.A., Zacharchuk, C.M. and Henkart, P.A. (1997) Target cell lysis by CTL granule exocytosis is independent of ICE/Ced-3 family proteases. Immunity 6(2), 209-15. Sarson, A.J., Abdul-Careem, M.F., Read, L.R., Brisbin, J.T. and Sharif, S. (2008) Expression of cytotoxicity-associated genes in Marek's disease virus-infected chickens. Viral Immunol 21(2), 267-72. Sharma, J.M. (1984) Effect of infectious bursal disease virus on protection against Marek's disease by turkey herpesvirus vaccine. Avian Dis 28(3), 629-40. Sharma, J.M., Dohms, J., Walser, M. and Snyder, D.B. (1993) Presence of lesions without virus replication in the thymus of chickens exposed to infectious bursal disease virus. Avian Dis 37(3), 741-8. Sharma, J.M., Dohms, J.E. and Metz, A.L. (1989) Comparative pathogenesis of serotype 1 and variant serotype 1 isolates of infectious bursal disease virus and their effect on humoral and cellular immune competence of specific-pathogen-free chickens. Avian Dis 33(1), 112-24. Sharma, J.M. and Fredericksen, T.L. (1987) Mechanism of T cell immunosuppression by infectious bursal disease virus of chickens. Prog Clin Biol Res 238, 283-94. 142 Sharma, J.M., Kim, I.J., Rautenschlein, S. and Yeh, H.Y. (2000) Infectious bursal disease virus of chickens: pathogenesis and immunosuppression. Dev Comp Immunol 24(2-3), 223-35. Sharma, J.M. and Lee, L.F. (1983) Effect of infectious bursal disease on natural killer cell activity and mitogenic response of chicken lymphoid cells: role of adherent cells in cellular immune suppression. Infect Immun 42(2), 747-54. Sijben, J.W., Klasing, K.C., Schrama, J.W., Parmentier, H.K., van der Poel, J.J., Savelkoul, H.F. and Kaiser, P. (2003) Early in vivo cytokine genes expression in chickens after challenge with Salmonella typhimurium lipopolysaccharide and modulation by dietary n--3 polyunsaturated fatty acids. Dev Comp Immunol 27(67), 611-9. Sivanandan, V. and Maheswaran, S.K. (1980a) Immune profile of infectious bursal disease (IBD). II. Effect of IBD virus on pokeweed-mitogen-stimulated peripheral blood lymphocytes of chickens. Avian Dis 24(3), 734-42. Sivanandan, V. and Maheswaran, S.K. (1980b) Immune profile of infectious bursal disease: I. Effect of infectious bursal disease virus on peripheral blood T and B lymphocytes of chickens. Avian Dis 24(3), 715-25. Sivanandan, V. and Maheswaran, S.K. (1981) Immune profile of infectious bursal disease. III. Effect of infectious bursal disease virus on the lymphocyte responses to phytomitogens and on mixed lymphocyte reaction of chickens. Avian Dis 25(1), 112-20. Skeeles, J.K., Lukert, P.D., De Buysscher, E.V., Fletcher, O.J. and Brown, J. (1979a) Infectious bursal disease viral infections. I. Complement and virus-neutralizing antibody response following infection of susceptible chickens. Avian Dis 23(1), 95-106. Skeeles, J.K., Lukert, P.D., De Buysscher, E.V., Fletcher, O.J. and Brown, J. (1979b) Infectious bursal disease viral infections. II. The relationship of age, complement levels, virus-neutralizing antibody, clotting, and lesions. Avian Dis 23(1), 107-17. Smyth, M.J. and Trapani, J.A. (1995) Granzymes: exogenous proteinases that induce target cell apoptosis. Immunol Today 16(4), 202-6. Snyder, D.B. (1990) Changes in the field status of infectious bursal disease virus. Avian Pathol 19(3), 419-23. Stoute, S.T., Jackwood, D.J., Sommer-Wagner, S.E., Cooper, G.L., Anderson, M.L., Woolcock, P.R., Bickford, A.A., Senties-Cue, C.G. and Charlton, B.R. (2009) The diagnosis of very virulent infectious bursal disease in California pullets. Avian Dis 53(2), 321-6. 143 Subler, K.A., Mickael, C.S. and Jackwood, D.J. (2006) Infectious bursal disease virusinduced immunosuppression exacerbates Campylobacter jejuni colonization and shedding in chickens. Avian Dis 50(2), 179-84. Takeda, K. and Akira, S. (2004) TLR signaling pathways. Semin Immunol 16(1), 3-9. Takeuchi, O. and Akira, S. (2009) Innate immunity to virus infection. Immunol Rev 227(1), 75-86. Tanimura, N. and Sharma, J.M. (1997) Appearance of T cells in the bursa of Fabricius and cecal tonsils during the acute phase of infectious bursal disease virus infection in chickens. Avian Dis 41(3), 638-45. Tanimura, N. and Sharma, J.M. (1998) In-situ apoptosis in chickens infected with infectious bursal disease virus. J Comp Pathol 118(1), 15-27. Tham, K.M. and Moon, C.D. (1996) Apoptosis in cell cultures induced by infectious bursal disease virus following in vitro infection. Avian Dis 40(1), 109-13. Trapani, J.A., Jans, D.A., Jans, P.J., Smyth, M.J., Browne, K.A. and Sutton, V.R. (1998) Efficient nuclear targeting of granzyme B and the nuclear consequences of apoptosis induced by granzyme B and perforin are caspase-dependent, but cell death is caspase-independent. J Biol Chem 273(43), 27934-8. Trapani, J.A. and Smyth, M.J. (2002) Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol 2(10), 735-47. Vakharia, V.N., Snyder, D.B., Lutticken, D., Mengel-Whereat, S.A., Savage, P.K., Edwards, G.H. and Goodwin, M.A. (1994) Active and passive protection against variant and classic infectious bursal disease virus strains induced by baculovirusexpressed structural proteins. Vaccine 12(5), 452-6. van den Berg, T.P., Eterradossi, N., Toquin, D. and Meulemans, G. (2000) Infectious bursal disease (Gumboro disease). Rev Sci Tech 19(2), 509-43. Van Parijs, L. and Abbas, A.K. (1996) Role of Fas-mediated cell death in the regulation of immune responses. Curr Opin Immunol 8(3), 355-61. Vasconcelos, A.C. and Lam, K.M. (1994) Apoptosis induced by infectious bursal disease virus. J Gen Virol 75 ( Pt 7), 1803-6. Vasconcelos, A.C. and Lam, K.M. (1995) Apoptosis in chicken embryos induced by the infectious bursal disease virus. J Comp Pathol 112(4), 327-38. Wahid, R., Cannon, M.J. and Chow, M. (2005) Virus-specific CD4+ and CD8+ cytotoxic T-cell responses and long-term T-cell memory in individuals vaccinated against polio. J Virol 79(10), 5988-95. 144 Ward, J.R., Bingle, L., Judge, H.M., Brown, S.B., Storey, R.F., Whyte, M.K., Dower, S.K., Buttle, D.J. and Sabroe, I. (2005) Agonists of toll-like receptor (TLR)2 and TLR4 are unable to modulate platelet activation by adenosine diphosphate and platelet activating factor. Thromb Haemost 94(4), 831-8. Werts, C., Girardin, S.E. and Philpott, D.J. (2006) TIR, CARD and PYRIN: three domains for an antimicrobial triad. Cell Death Differ 13(5), 798-815. Winterfield, R.W., Fadly, A.M. and Bickford, A. (1972) Infectivity and distribution of infectious bursal disease virus in the chicken. Persistence of the virus and lesions. Avian Dis 16(3), 622-32. Withers, D.R., Young, J.R. and Davison, T.F. (2005) Infectious bursal disease virusinduced immunosuppression in the chick is associated with the presence of undifferentiated follicles in the recovering bursa. Viral Immunol 18(1), 127-37. Wyeth, P.J. and Cullen, G.A. (1978) Susceptibility of chicks to infectious bursal disease (IBD) following vaccination of their parents with live IBD vaccine. Vet Rec 103(13), 281-2. Wyeth, P.J., O'Brien, J.D. and Cullen, G.A. (1981) Improved performance of progeny of broiler parent chickens vaccinated with infectious bursal disease oil-emulsion vaccine. Avian Dis 25(1), 228-41. Yao, K., Goodwin, M.A. and Vakharia, V.N. (1998) Generation of a mutant infectious bursal disease virus that does not cause bursal lesions. J Virol 72(4), 2647-54. Yeh, H.Y., Rautenschlein, S. and Sharma, J.M. (2002) Protective immunity against infectious bursal disease virus in chickens in the absence of virus-specific antibodies. Vet Immunol Immunopathol 89(3-4), 149-58. Yokota, S., Okabayashi, T. and Fujii, N. (2010) The battle between virus and host: modulation of Toll-like receptor signaling pathways by virus infection. Mediators Inflamm 2010, 184328. Yoneyama, M., Kikuchi, M., Natsukawa, T., Shinobu, N., Imaizumi, T., Miyagishi, M., Taira, K., Akira, S. and Fujita, T. (2004) The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 5(7), 730-7. Yuan, L., Wen, K., Azevedo, M.S., Gonzalez, A.M., Zhang, W. and Saif, L.J. (2008) Virus-specific intestinal IFN-gamma producing T cell responses induced by human rotavirus infection and vaccines are correlated with protection against rotavirus diarrhea in gnotobiotic pigs. Vaccine 26(26), 3322-31. 145