* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Maine EMS Medivax EMS Influenza Vaccination Program
Survey
Document related concepts
Transcript
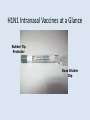
Maine EMS Medivax EMS Vaccination Program Objective • To develop a protocol and process to allow licensed MEMS ALS personnel already trained to administer medications via the IM/SC/intranasal route to provide immunizations in an organized event authorized by a licensed Physician Objectives • To be able to support / deliver vaccinations as force protection to essential personnel and the capabilities to deliver vaccination to the public is need is determined. • To teach and instruct Maine EMS personnel, including the Emergency EMT Basic Level on the safe and proper delivery of vaccinations via the Intranasal Route. Basic EMTs who have completed the Maine EMS MediVax program are allowed to perform intranasal H1N1 vaccine in the 2009/2010 season with the caveat that an ALS resource or equivalent (nurse physician, or NP/PA) with the resources to care for adverse effects (including allergic reactions) is available on scene. A basic service cannot host a vaccine clinic individually but Basic EMTs can be used under the auspices of a larger physician proscripted event to act as a resource for administering IN vaccine. EMS Agency Requirements • MEMS licensed service permitted at or above the intermediate level • The service immunization program and the instructor for the Maine MEDIVAX program must both be approved by the service Medical Director • Service must maintain all records for any vaccinations provided by the service to their personnel. Service Medical Director • EMS Services that want to provide vaccinations to their own personnel must have a Medical Director who authorizes the program. • If the service does not have a service Medical Director, then they should approach a local Health Care Facility or the Regional Medical Director for assistance. Sponsoring Agency Requirements • Program must be authorized by a licensed Physician. • Sponsoring Organization is required to provide all paperwork and maintain records for 7 years post vaccination. EMS Provider Requirements • MEMS licensed Basic EMT (IN vaccine only)Intermediate, Critical Care, or Paramedic. • Completed an approved* MEMS Vaccination Administration training program. * An approved program is one where the program and instructor have been approved by the Service Medical Director. Liability • EMS Providers providing immunizations need to check with the sponsoring organization about liability coverage. • Liability coverage may differ based upon changes in state/federal law, pandemic declarations, and employer coverage Governor’s Executive Order Proclamation; Mission and Scope; E. To the extent necessary to assure the timely provision of seasonal influenza and 2009 Influenza A (H1N1) vaccination in accordance with the guidance of the federal Centers for Disease Control and Prevention, the Maine Center for Disease Control and the Department of Education, the Maine Emergency Management Agency will exercise its authority pursuant to 37-B M.R.S.A. 784-A to designate appropriate health care workers licensed in the State and authorized to administer influenza vaccines to participate in the vaccination clinics in accordance with the requirements of this Proclamation. Liability F. All persons designated by the Maine Emergency Management Agency to participate in the vaccine administration pursuant to Paragraph E shall. pursuant to 37-B M.R.S.A. 784-A, be deemed to be an employee of the State and entitled to immunity pursuant to 37-B M.R.S.A 822. Training Overview • IM injection training (NHTSA National Standard Curriculum) • Video Overview • Intranasal training • Administrative Requirements – Handbook – Lecture Intranasal Administration • Nasal medication delivery takes a middle path between slow onset oral medications and invasive, highly skilled delivery of intravenous medications. Because the nasal mucosa is highly vascularized, delivery of a thin layer of medication across a broad surface area can result in rapid transmucosal absorption of the medication into the blood stream and cerebral spinal fluid. Procedure Instruction of Intranasal Medication Administration H1N1 Intranasal Vaccines at a Glance H1N1 Intranasal Vaccines at a Glance Rubber Tip Protector Dose Divider Clip H1N1 Intranasal Vaccines at a Glance Clip in middle of medication plunger is known as Dose Divider Clip Procedure Influenza 2009 (Inactivated) Vaccine AKA, The Seasonal Flu Influenza 2009 (Inactivated) Vaccine Serious problems from influenza vaccine are very rare. The viruses in inactivated influenza vaccine have been killed, so you cannot get influenza from the vaccine. Influenza 2009 (Inactivated) Vaccine Mild problems: •soreness, redness, or swelling where the shot was given •hoarseness, sore or red eyes, cough, itchiness •fever •aches If these problems occur, they usually begin soon after the shot and last 1 to 2 days. Influenza 2009 (Inactivated) Vaccine Severe problems: Life-threatening allergic reactions from vaccines are very rare. If they do occur, it is usually within a few minutes to a few hours after the shot. Influenza 2009 (Inactivated) Vaccine It is important to differentiate other, more common, nonallergic clinical syndromes from anaphylactic vaccine reactions. Vasovagal reactions with pallor, bradycardia, weakness, dizziness, and brief syncope may occur five to 15 minutes after vaccination. These reactions occur more often in adolescents and adults than in children. 2009 H1N1 (Inactivated) Vaccine AKA, The Swine Flu 2009 H1N1 (Inactivated) Vaccine A vaccine, like any medicine, could possibly cause serious problems, such as severe allergic reactions. The risk of a vaccine causing serious harm, or death, is extremely small. 2009 H1N1 (Inactivated) Vaccine Serious problems from influenza vaccine are very rare. The viruses in inactivated influenza vaccine have been killed, so you cannot get influenza from the vaccine. 2009 H1N1 (Inactivated) Vaccine Mild problems: •soreness, redness, or swelling where the shot was given •hoarseness, sore or red eyes, cough, itchiness •fever •aches If these problems occur, they usually begin soon after the shot and last 1 to 2 days. 2009 H1N1 (Live, Attenuated) Vaccine AKA, The Swine Flu ANAPHLAXIS / ALLERGIC REACTIONS REVIEW Allergic Reaction An exaggerated reaction by the body’s immune system to any substance ANAPHLAXIS / ALLERGIC REACTIONS REVIEW Anaphylaxis A life-threatening allergic reaction which causes shock (hypoperfusion) and airway swelling ANAPHLAXIS / ALLERGIC REACTIONS REVIEW ALERT!!!! The PEDIACTRIC Population that we will be working with, have the potential of a delayed anaphylactic reaction after the vaccine. Reactions can occur up to 15 minutes later! Signs and Symptoms Skin Itching Hives Flushing Warm, tingling feeling Swelling (especially face, neck, hands, feet, tongue) Signs and Symptoms Respiratory Tightness in throat/chest Cough Rapid, labored, noisy breathing Hoarseness Stridor and wheezing Signs and Symptoms Cardiac Increased heart rate Low blood pressure Signs and Symptoms Generalized Findings Itchy, watery eyes and runny nose Headache Sense of impending doom Signs and Symptoms Decreasing mental status Signs and symptoms of shock (hypoperfusion) or respiratory distress TREATMENT • TREAT AS PER PROTOCOL MAINE EMS GOLD 1 Quick PEDI Drug Reference Chart Protocol • Complete vaccine administration record (VAR) and have recipient sign it • Distribute a current season Vaccine Information Statement (VIS) • Administer the vaccination via IM/SC/intranasal route per the drug package insert or the instructions of the Medical Director Documentation Overview 1. Vaccination records will be maintained by the agency for 7 years 2. Sponsoring agencies will provide vaccinated individuals with copies of their vaccination administration records (VAR) on request 3. All VAR’s will be reviewed by the sponsoring agency medical director or their designee Documents 1. Vaccine Administration Record (VAR) 2. Vaccine Information Sheet (VIS) 3. Vaccine Adverse Event Reposting System (VAERS) Vaccine Administration Record • Tracking and consent documentation • Identifies risks and contraindications to vaccine • Requires patient review and signature • Documents the vaccination – MFR lot # – Dose route site – Provider Vaccine Information Statement (VIS) • http://www.cdc.gov/vaccines/pubs/vis/downloads/vis-flu.pdf • Patient information regarding the vaccine • To be reviewed by patient before vaccination Vaccine Adverse Event Reporting System (VAERS) • National program that monitors the safety of vaccines after they are licensed • VAERS is used to watch and record patient “adverse events” • Beware that often patient reported problems are unrelated to vaccination – EG. Contracting a cold after having an influenza vaccine Vaccine Adverse Event Reporting System (VAERS) What events should be reported? • Any serious problem that occurs after administration of a vaccine Vaccine Adverse Event Reporting System (VAERS) How do I report? • Hotline – (800) 822-7967 • Internet – Secure.vaers.org/vaersdataentryintro.htm • Printable form – www.vaers.hhs.gov – VAERS, PO Box 1100, Rockville, MD 20849-1100 Review • Organized, Physician-prescripted event only • Complete vaccine administration record (VAR) • Distribute Vaccine Information Statement (VIS) • Perform the vaccination via IM injection • Report serious post vaccination problems using VAERS QUESTIONS??? References • • • • • • • Nursing Interventions and Clinical Skills, 3rd Ed., Mosby © 2000 www.webmd.Com www.google.Com (Images) Maine EMS Medivax Program Update August 2009 MAINE EMS PREHOSPITAL TREATMENT PROTOCOLS, JULY 2008 Limmer, Emergency Care Update, 10th Ed, 2007, Pearson Education. Inc. MedImmune, H1N1 (Live Attenuated) Intranasal Spray Vaccine Drug Insert Fact Sheet Acknowledgements • • • • • Dan Batsie, EMT-P, Northeast EMS Rick Petrie, EMT-P, Kennebec Valley & Northeast EMS Lt. Nate Contreras, Scarborough Fire Dept. Steve Diaz, MD, Maine EMS Medical Director Matt Sholl, MD, Asst. Maine EMS Medical Director