* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download External compression of superior vena cava after the replacement of
Management of acute coronary syndrome wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Echocardiography wikipedia , lookup
Aortic stenosis wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
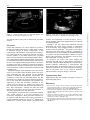
European Journal of Echocardiography (2008) 9, 589–590 doi:10.1093/ejechocard/jen116 External compression of superior vena cava after the replacement of ascending aorta Johannes Wacker, George Djaiani, Rita Katznelson*, and Jacek Karski Received 17 September 2007; accepted after revision 9 February 2008; online publish-ahead-of-print 1 May 2008 KEYWORDS SVC obstruction; HOCM We present a rare complication after open-heart surgery resulting in compression of the superior vena cava (SVC) with the concurrent findings of the hypertrophic obstructive cardiomyopathy physiology. A 59-year-old woman developed a low cardiac output syndrome, persistent hypotension, and increasing filling pressures after emergency replacement of the ascending aorta and resuspension of the aortic valve due to a type A aortic dissection. Transesophageal echocardiography (TEE) evaluation revealed partial SVC obstruction, under-filled left ventricle (LV), and a persistent mitral systolic anterior motion with increasing pressure gradient in the left ventricular outflow tract (LVOT). Surgical exposure uncovered an intrapericardial thrombus around the aortic graft compressing the SVC. Removal of the thrombus resulted in immediate haemodynamic improvement and elimination of both SVC and LVOT obstructions. A comprehensive TEE exam should always be performed, and all the structures should be visualized for the proper diagnosis and management of patients after cardiac surgery. Clinical course A 59-year-old woman was admitted to the hospital with hypotension, chest pain, and shortness of breath. The transesophageal echocardiographic examination (TEE) revealed pericardial effusion, a sigmoid basal ventricular septum, severe posteriorly directed mitral regurgitation (MR), mitral systolic anterior motion (SAM), turbulent flow in the left ventricular outflow tract (LVOT), and a type A aortic dissection with an intimal flap extending from the aortic root to the mid-arch. Emergency surgery consisted of replacement of the ascending aorta and resuspension of the aortic valve. Postoperative transesophageal echocardiography (TEE) showed mild MR. After surgery, patient was transferred to the intensive care unit for elective postoperative ventilation and haemodynamic monitoring. In the first 6 postoperative hours, patient’s condition was complicated by a low cardiac output syndrome necessitating inotropic and vasopressor support. Over a period of 3 h, the central venous pressure increased from 8 to 22 mmHg, and the pulmonary artery diastolic pressure increased from 10 to 15 mmHg. Furthermore, persistent hypotension despite fluid administration and increasing filling pressures led to the clinical impression of cardiac tamponade. A TEE * Corresponding author. Tel: þ1 416 439 4800, ext 2840; fax: þ1 416 340 3698. E-mail address: [email protected] demonstrated an under-filled, thickened hyperdynamic LV. Basal intraventricular septal thickness was 22 mm. The distance from the right coronary cusp of the aortic valve to the contact point of the anterior mitral valve leaflet with the ventricular septum was 17 mm. A persistent SAM (Supplementary data online, Video-clip S1) with the LVOT pressure gradient of 120 mmHg was noted. Narrowing of the superior vena cava (SVC) on 2D imaging (Figure 1) and a turbulent flow from the SVC to right atrium (RA) were also noted (Supplementary data online, Video-clip S2). The SVC diameter was 5.3 mm and looked narrowed. This finding was suggestive of external compression of the SVC consistent with either thrombus or haematoma. There was no evidence of external compression of RA or right ventricle (RV). Right ventricle appeared mildly hypokinetic. Moderate eccentric MR and moderate central tricuspid regurgitation (TR) were also identified. The patient was taken back to the operating room for chest exploration. Surgical exposure uncovered an intrapericardial thrombus around the aortic graft compressing the SVC. Removal of the clot resulted in widening of the SVC measured at 13 mm (Figure 2) and a laminar SVC flow. The LVOT pressure gradient dropped to 27 mmHg, and right ventricular function improved. Cardiac function continued to improve and the patient was discharged 12 days after surgery. A transthoracic echocardiogram before discharge showed mild centrally Published on behalf of the European Society of Cardiology. All rights reserved. & The Author 2008. For permissions please email: [email protected]. Downloaded from http://ehjcimaging.oxfordjournals.org/ at Pennsylvania State University on March 3, 2014 Department of Anesthesia, Toronto General Hospital, University Health Network, University of Toronto, 200 Elizabeth Street, Toronto, Ontario, Canada M5G 2C4 590 J. Wacker et al. Figure 2 A 2D view of superior vena cava (SVC) after chest exploration and removal of haematoma compressing the SVC. directed MR and TR and trivial circumferential pericardial effusion. pressure, and equalization of central pressures, and is a clinical diagnosis, which can be supported and/or confirmed by echocardiography. Although a few case reports have described localized haematoma post cardiac surgery resulting in compression of the RA and SVC, they commonly describe a clinical presentation of the SVC syndrome.3,4 We present a rare complication after open heart surgery resulting in compression of the SVC with the concurrent findings of the HOCM physiology in a patient with sigmoid basal septum. Without a comprehensive TEE examination, this diagnosis could not have been established in a timely manner. In conclusion, the current case report supports our contention that TEE offers a comprehensive evaluation of the cardiac tissues and helps delineate the causes of haemodynamic instability. Furthermore, a comprehensive TEE exam should always be performed, and all the structures should be visualized for the proper diagnosis and management of the patient. This, in turn, helps to determine whether medical or surgical therapy is warranted. Discussion Prompt TEE evaluation is of critical importance in haemodynamically unstable patients after cardiac surgery leading to significant changes in patient management.1 In the reported case, TEE evaluation revealed partial SVC obstruction, under-filled LV, and a persistent SAM with increasing pressure gradient in the LVOT. Patients with a sigmoid basal ventricular septum may develop hypertrophic obstructive cardiomyopathy (HOCM) physiology which is particularly accentuated in the presence of under-filled or hyperdynamic LV due to the ongoing bleeding (hypovolaemia), and inotropic support. This type of response is related to the pressure gradient between the apical and basal parts of the LV during the ejection phase creating the obstruction of LVOT with SAM followed by MR. Expeditious surgical chest exploration led to the identification of an intrapericardial thrombus around the aortic graft compressing the SVC. Removal of the thrombus resulted in immediate haemodynamic improvement and elimination of both SVC and LVOT obstructions. The SVC obstruction may explain the patient’s delayed responsiveness to the initial fluid resuscitation and emphasizes the importance of early TEE evaluation. Utilization of the inferior vena cava access (e.g. femoral vein) rather than SVC could have potentially facilitated the effectiveness of early fluid resuscitation. Although the SAM with LVOT obstruction was present during the initial procedure, it was likely aggravated by the SVC obstruction. Haemodynamic compromise due to extracardial effusions after cardiac surgery is a rare but potentially livethreatening complication. While pericardial effusions are as common as 85% after open heart surgery, progression to cardiac tamponade ranges from 0.15 to 6%.2 Tamponade is suspected by reduced cardiac output, decreased blood Supplementary data Supplementary data are available at European Journal of Echocardiography online. References 1. Wake PG, Ali M, Carroll J, Siu SC, Cheng DC. Clinical and echocardiographic diagnoses disagree in patients with unexplained hemodynamic instability after cardiac surgery. Can J Anaesth 2001;48:778–83. 2. Kuvin JT, Harati NA, Pandian NG, Pandian NG, Bojar RM, Khabbaz KR. Postoperative cardiac tamponade in the modern surgical era. Ann Thorac Surg 2002;74:1148–53. 3. Aebischer N, Shurman AJ, Sharma S. Late localized tamponade causing superior vena cava syndrome: an unusual complication of aortic valve replacement. Am Heart J 1988;115:1130–2. 4. Pierli C, Iadanza A, Del Pasqua A, Fineschi M. Acute superior vena cava and right atrial tamponade in an infant after open heart surgery. Int J Cardiol 2002;83:195–7. Downloaded from http://ehjcimaging.oxfordjournals.org/ at Pennsylvania State University on March 3, 2014 Figure 1 A black and white print of a colour flow Doppler view reflecting the narrowing of superior vena cava.