* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Atom - Alvin ISD
Survey
Document related concepts
Transcript
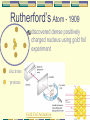
Atomic Model Development Rev 6/7/06 Democritus – 400 BC Defined an Atom as the smallest unit of a material that can not be split up Greek word “atom” means invisible Atoms are solid, homogeneous, indestructible and indivisible Dalton’s Atom - 1807 Atom = Solid sphere All elements are composed of tiny invisible particles called atoms Atoms of same element- alike & different from atoms of other elements Atoms join together in whole number ratios to form compounds..that means they join in a definate proportion every time the same compound is formed. Henri Becquerel 1896 Discovered radioactivity Radiation produced spontaneously by unstable atomic nucleus Thomson’s Atom - 1897 Discovered electrons Plum Pudding model (for us Americans it looks like a chocolate chip cookie model) + charge “pudding” Electrons - charge Rutherford’s Atom - 1909 discovered dense positively charged nucleus using gold foil experiment electrons protons Gold Foil Animation James Chadwick 1932 •Identified neutron •Same mass as proton •No charge Bohr’s atom - 1913 electrons orbit in energy levels around the protons in the nucleus. p+ like our solar system… (electrons the planets, the nucleus the sun!) Atomic Time Line review…