* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Molecular orbital approach to bonding in octahedral complexes, ML 6
Hydroformylation wikipedia , lookup
Cluster chemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metal carbonyl wikipedia , lookup
Metalloprotein wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Spin crossover wikipedia , lookup
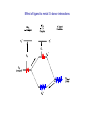
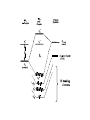
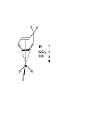
Molecular orbital theory approach to bonding in transition metal complexes Molecular orbital (MO) theory considers the overlap of atomic orbitals, of matching symmetry and comparable energy, to form molecular orbitals. When atomic orbital wave functions are combined, they generate equal numbers of bonding and antibonding molecular orbitals. The bonding MO is always lower in energy than the corresponding antibonding MO. Electrons occupy the molecular orbitals in order of their increasing energy in accordance with the aufbau principal. Bond-Order = Electrons in bonding MOs – Electrons in antibonding MOs 2 Molecular orbital descriptions of dioxygen species. Molecular orbital approach to bonding in octahedral complexes, ML6 ______________________________________________________________________________________________________________________________ Combinations of atomic orbitals Molecular Orbital 4s ± 1/√6(σ1 + σ2 + σ3 + σ4 + σ5 + σ6) a1g 4px ± 1/√2 (σ1 σ2) 4py ± 1/√2 (σ3 σ4) 4pz ± 1/√2 (σ5 σ6) t1u 3dx2 - y2 ± 1/2 (σ1 + σ2 σ3 σ4) 3dz2 ± 1/√12 (2 σ5 + 2 σ6 σ1 σ2 σ3 σ4) eg 3dxy 3dxz 3dyz t2g Non-bonding in σ complex _______________________________________________________________________________________________ MO diagram for s-bonded octahedral metal complex M.O. Diagram for Tetrahedral Metal Complex Since the metal 4p and t2 orbitals are of the same symmetry, e → t2 transitions in Td complexes are less “d-d” than are t2g → eg transitions in Oh complexes. They are therefore more allowed and have larger absorbtivity values (e) Metal-ligand P-bonding interactions t2g orbitals (dxy, dxz, dyz) are non-bonding in a s-bonded octahedral complex ligands of P-symmetry overlap with the metal t2g orbitals to form metal-ligand P-bonds. P-unsaturated ligands such as CO, CN- or 1,10-phenanthroline or sulfur and phosphorus donor ligands (SR2, PR3) with empty t2g-orbitals have the correct symmetry to overlap with the metal t2g orbitals. Pacceptor interactions have the effect of lowering the energy of the non-bonding t2g orbitals and increasing the magnitude Doct. This explains why P-acceptor ligands like CO and CN- are strong field ligands, and why metal carbonyl and metal cyanide complexes are generally low-spin. P-interactions involving P-donation of electron density from filled porbitals of halides (F- and Cl-) and oxygen donors, to the t2g of the metal, can have the opposite effect of lowering the magnitude of Doct. In this case, the t2g electrons of the s-complex, derived from the metal d orbitals, are pushed into the higher t2g* orbitals and become antibonding. This has the effect of lowering Doct. M Metal- d (t2g) L Ligand p (full) e.g. halide ion, XRO- Ligand - p Effect of ligand to metal Pdonor interactions P-alkene organometallic complexes Zeise’s Salt, K[PtCl3(C2H4)] Pacceptor interactions have the effect of lowering the energy of the non-bonding t2g orbitals and increasing the magnitude Doct. This lowering of the energy of the t2g orbitals also results in 9 strongly bonding M.O.’s well separated in energy from the antibonding orbitals Consequences of P-bonding interactions between metal and ligand Enhanced D-splitting for P-acceptor ligands makes P-unsaturated ligands like CO, CN- and alkenes very strong-field ligands. Stabilization of metals in low oxidation states. Delocalization of electron density from low oxidation state (electron-rich) metals into empty ligand orbitals by “back-bonding” enables metals to exist in formally zero and negative oxidation states (Fe(CO)5, Ni(CO)42-). Accounts for organometallic chemistry of P-Acid ligands The application of the “18-electron rule” to predict and rationalize structures of many Pacid organometallic compounds. Electron donation by P-unsaturated ligands Examples of 18-electron organometallic complexes with Punsaturated (P-acid) ligands Scope of 16/18-electron rules for d-block organometallic compounds Usually less than 18 electrons Usually 18 electrons 16 or 18 Electrons Sc Y Cr Mo W Co Rh Ir Ti V Zr Nb Mn Tc Re Fe Ru Os Ni Pd Pt Metal-ligand interactions involving bonding and antibonding molecular orbitals of O2 *of O2 (empty) of O2 (filled) * O O O O Fe dz2 of Fe (empty) Fe t2g (dxz ,dyz ) of Fe (filled)