* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PowerPoint Presentation - Slide 1
Survey
Document related concepts
Transcript
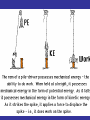
Work & Energy "There is nothing new to be discovered in physics now, All that remains is more and more precise measurement.” -Lord Kelvin “Time is nature’s way of making certain that everything doesn't happen all at once.” -Woody Allen Some Kinds of Energy • • • • Kinetic Energy – the energy of moving objects Heat, or thermal energy – of warm bodies. Chemical Energy – of chemical reactions. Gravitational Potential Energy – of a gravitational field. • Electromagnetic Energy – energy associated with electric and magnetic forces. • Mass Energy – all objects have energy by virtue of their mass, the energy released in nuclear explosions. Kinetic Energy This is “moving” energy Marion Jones Sprints to Victory in the 200 meter. Gravitational Potential Energy: This is “stored” energy something has when it is above the surface of the Earth. Example of Potential Energy Cheryl Haworth lifts 135.2 kg a distance of 2 meters Mass and Energy One of Einstein’s many contributions was the recognition that mass is simply another form of energy. Mass and other forms of energy can be interchanged, as can kinetic energy, potential energy, heat, etc. The amount of energy contained in an object with mass m is given by the famous equation E = mc2 where c is the speed of light. The speed of light is equal to c = 3108 meters/second. Conservation of Energy • Energy is neither created nor destroyed but only transformed from one form to another. • In a closed system, the total amount of energy is conserved. If we add up the amount of energy in a closed system including all of the different forms, the sum will not change with time. • The total amount of energy never changes, it only moves from place to place and from one form to another. • Conservation of Energy applies not just to kinetic and potential energy, as in the example, but to all kinds of energy (heat, chemical, …) Conservation of Energy m = 50.9 kg Work and Energy Definition: Work is the energy added to an object through the action of forces over a distance. Work = Force x distance W = Fxd Joules = Newton x meters James Prescott Joule (1818-1889) Joule determined the mechanical equivalent of heat by measuring change in temperature produced by the friction of a paddlewheel attached to a falling weight. He made a series of measurements and found that, on average, a weight of 772 pounds falling through a distance of one foot would raise the temperature of one pound of water by 1° F. This corresponds to (772 ft lbs)(1.356 J/ft lb) = 59 453.6 Calories, or 1 cal = 4.15 Joules, in close agreement with the current accepted value of 1 calorie = 4.184 Joules. The son of a prosperous brewer in North England, Joule financed his experiments himself. Power The rate of doing work or expending energy P = Energy/Time Rock climbers gain a lot of potential energy but do so slowly, at low power Power Training Cyclists do work more quickly than rock climbers. They expend more power. Cadel Evans expends about 10,000 Calories in a 2 hour race. This corresponds to a power of roughly 6 kilowatts. Racing: Tour de France Training in Arizona Niagara Falls 570,000 kg of water goes over the falls every second. The falls height is 55 meters. Thus the potential energy of 1 kg of water is E=mha=19.855 J = 539 J. The total power is then P=570,000539 Joules/sec=3.1108 Watts or 310 Megawatts. Climbing out of the Grand Canyon How big a lunch is needed? Energy from lunch = Work to be done W= = = = mgh (62 kg)(9.82m/s2)(1500 m) 9.1x105 Joules 218 Calories That is, 1 peach & a glass of milk That’s it? Nope – we’re inefficient Humans work at roughly 15% efficiency. The rest of the energy goes into heat and non-work productive movement. So, to climb the Grand Canyon, we need 1450 Cal ! Two club sandwiches, one egg, 1 fruit and 1 glass of milk.