* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download INTRODUCTION: - PharmaStreet
Neuropharmacology wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Plateau principle wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Prescription costs wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Hyaluronic acid wikipedia , lookup
Drug discovery wikipedia , lookup
Drug design wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
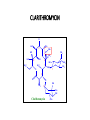
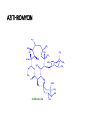
O H3C CH3 H3C OH HO CH3 H3C HO N OH H3C O O O CH3 CH3 O O CH3 O O CH3 H3C CH3 Erythromycin OH CH3 • Belong to the Polyketide (produced by fungi) class of natural products. • A group of antibiotics consisting of a macrolide ring • A large lactone ring to which one or more deoxy sugars, are attached. • The lactone ring can be either 14, 15 or 16 membered. • MACROLIDES:• • • • ERYTHROMYCIN CLARITHROMYCIN AZITHROMYCIN ROXITHROMYCIN ERYTHROMYCIN • Isolated from Streptomyces erythreus in 1952 • A drug of choice and an alternative to penicillin in individuals who are allergic to β-lactam antibiotics • Slightly water soluble • Stable in aqueous solutions at or below room temperature • Unstable in acidic or basic conditions or at high temperatures. • Inhibits protein synthesis • By reversibly binding to the 50S ribosomal subunit • Suppression of RNA-dependent protein synthesis by inhibition of translocation of mRNA • Typically bacteriostatic activity • Bactericidal at high concentrations against very susceptible organisms • More active in alkaline medium • After peptide bond formation between the newly attached amino acid and the nacent peptide chain at the acceptor (A) site the elongated peptide is translocated back to the peptidyl (P) site, making the A site available for next aminoacyl tRNA attachment. • This is prevented by erythromycin and the ribosome fails to move along the mRNA to expose the next codon. • As an indirect consequence, peptide chain may be prematurely terminated: synthesis of larger proteins is specifically suppressed. Mechanism of action ANTIMICROBIAL SPECTRUM :• Narrow spectrum • Mostly gram-positive • Few gram-negative bacteria • Highly active against:- Str. pyogenes and Str. pneumoniae, N. gonorrhoeae, Clostridia, C. diphtheriae, Listeria. • Sensitive :penicillin-resistant Staphylococci and Streptococci were • Highly sensitive that are not affected by penicillin:- Campylobacter, Legionella, Branhamella catarrhalis, Gardnerella vaginalis and Mycoplasma. • Moderately sensitive :- H. influenzae, H. ducreyi, B. Pertussis,Chlamydia trachomatis, Str. viridans, N. Meningitidis and Rickettsiae RESISTANCE • Becoming a serious problem • Several mechanisms are: • Less permeable • Presence of efflux pump • Decreased affinity by plasmid encoded methylase enzyme • Presence of plasmid associated erythromycin esterase • Change in the 50S ribosome by chromosomal mutation Cross-resistance occurs between all macrolides PHARMACOKINETICS • Erythromycin is acid labile but all others (newer) are stable • Given as enteric coated tablets • Food delays absorption • Its acid esters are better absorbed • Widely distributed in the body except CSF • Diffuses into prostatic fluid, accumulates in macrophages • Concentrates in liver • Inflammation allows greater tissue penetration • 70-80% plasma protein bound • Renal excretion is minor (5%) • Its plasma t1/2 is 1.5hr, but it persists longer in tissues ADVERSE EFFECTS 1 . Gastrointestinal • Mild-to-severe epigastric pain • Diarrhoea 2. Reversible hearing impairment-Very high doses 3. Hypersensitivity • Rashes and fever are infrequent. 4. cholestatic jaundice (caused by thickened bile or bile plugs in the small biliary passages of the liver) INTERACTION • Erythromycin inhibits hepatic oxidation of many drugs • rise in plasma levels of theophylline, carbamazepine, valproate, ergotamine and warfarin. • To inhibition of CYP3A4 by erythromycin/ clarithromycin • Q-T prolongation, serious ventricular arrhythmias and death • due resulting in high blood levels of concurrently administered terfenadine (allergic inflammation of the nasal airways) / astemizole ( antihistamine drug)/ cisapride (treatment of gastroesophageal reflux disease). INTERACTIONS USES A . As an alternative to penicillin 1. Streptococcal pharyngitis, tonsillitis, mastoiditis and community acquired respiratory infections caused by pneumococci and H. influenzae respond equally well to erythromycin. • It is an alternative drug for prophylaxis of rheumatic fever and SABE. 2. Diphtheria (causing inflammation of the mucous membranes, ) 3. Tetanus (marked by rigidity and spasms of the voluntary muscles) 4. Syphilis and gonorrhoea 5. Leptospirosis B. As a first choice drug for 1 . Atypical pneumonia caused by Mycoplasma pneumoniae 2. Whooping cough:- treatment for eradicating B. pertussis from upper respiratory tract. 3. Chancroid C. As a second drug choice: 1. 2. 3. 4. Campylobactor enteritis Legionnaire’s pneumonia Chlamydia trachomitis Pencillin resistant Staphylococcal infections PROBLEMS WITH ERYTHROMYCIN • Acid labile • Narrow spectrum • Poor GI tolerance • Short elimination half-life • Gastric acid liability • Low oral bioavailability • Poor tissue penetration • Advantages of New macrolides • • • • • Broader spectrum Orally effective High blood concentration Longer t 1/2 Less toxicity ROXITHROMYCIN • Semisynthetic long-acting • Acid-stable • Antimicrobial spectrum resembles with erythromycin. • More potent against • Branh. catarrhalis, gard. vaginalis and Legionella • less potent against • B. pertussis. • Good eternal absorption • Good tissue penetration • Plasma t1/2 - 12 hr • Better gastric tolerability • Affinity for cytochrome P450 is lower, • Drug interactions are not ruled out. • An alternative to erythromycin • For respiratory, ENT, skin and soft tissue and genital tract infections with similar efficacy. ROXITHROMYCIN CLARITHROMYCIN O H3C CH3 9 CH3 O HO CH3 CH3 H3C OH 12 H3C N 6 HO 5 H3C O O O 1 1` 3 O O OH CH3 1`` CH3 OH O Clarithromycin CH3 CH3 CH3 CLARITHROMYCIN • Antimicrobial spectrum similar to erythromycin • In addition includes • • • • • Mycobact. avium complex (MAC), other atypical mycobacteria, Mycobact. leprae and some anaerobes but not Bact. Jragilis. • More active against • • • • • sensitive strains of gram-positive cocci, Moraxella, Legionella, Mycoplasma pneumoniae Helicobacter pylori. • Bacteria resistance to erythromycin are resistant to clarithromycin also. • More acid-stable • Rapidly absorbed • Oral bioavailability is -50% due to first pass metabolism • Food delays but does not decrease absorption. • Greater tissue distribution than erythromycin • Plasma t1/2 of 6-9 hours • An active metabolite is produced • Excreted unchanged in urine INDICATIONS • • • • • • Upper and lower RTI Sinusitis Otitis media Whooping cough Pneumonia Skin and skin structure infections due to Strep. pyogenes and some Staph. aureus. • First line drug in combination regimens for MAC infection in AIDS patients • Second line drug for other mycobacterial diseases as well as leprosy SIDE EFFECTS • Similar to erythromycin, • But gastric tolerance is better. • High doses can cause reversible hearing loss. • Pseudomembranous enterocolitis (inflammation of the large intestine) • Hepatic dysfunction or rhabdomyolysis (the destruction of striated muscle cells). • Drug interaction is similar to erythromycin. AZITHROMYCIN H3C N CH3 H3C OH HO CH3 CH3 12 H3C OH N HO 5 H3C O O O 1 CH3 1` 3 O O CH3 OCH3 1`` CH3 OH O Azithromycin CH3 CH3 CH3 AZITHROMYCIN • Expanded spectrum • Improved pharmacokinetics • Better tolerability • Improved drug interaction profiles SPECTRUM:• More active than other macrolides against H. influenzae, • But less active against gram-positive cocci • High activity on respiratory pathogens• Mycoplama, • Chlamydia pneumoniae, • Legionella, • Moraxella • others like Campylobacter, Ch. Trachomatis, H. ducreyi, Calymm, granulomatis, N. gonorrhoea • Not active against erythromycin resistant bacteria • Penicillinase producing St. aureus are inhibited. • Good activity is against MAC PHARMACOKINETICS:• Acid-stability, • Rapid oral absorption • Marked tissue distribution • Intracellular penetration • T1/2 of >50 hr. • Largely excreted in bile • Renal excretion is - 10% INDICATIONS • As first choice over erythromicin (a) Legionnaires' pneumonia (b) Chlamydia trachomatis (c). Donovanosis (characterized by ulcerative genital lesions) caused by Calymmatobacterium granulomatis • The other indications :• • • • • • • Pharyngitis, Tonsillitis, Sinusitis, Otitis media, Pneumonias, Chronic bronchitis, Streptococcal and some staphylococcal skin and soft tissue infections. • Effective in the prophylaxis and treatment of MAC in AIDS patients. • Other potential uses are in • Typhoid, • Oxoplasmosis • Malaria. SIDE EFFECTS •Mild gastric upset, •Abdominal pain (less than erythromycin), •Headache •Dizziness. •Not affecting hepatic CYP3A4 enzyme. THANK YOU - PHARMA STREET