* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download What is HCV?
Oesophagostomum wikipedia , lookup
Microbicides for sexually transmitted diseases wikipedia , lookup
Neonatal infection wikipedia , lookup
Ebola virus disease wikipedia , lookup
Diagnosis of HIV/AIDS wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Influenza A virus wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Orthohantavirus wikipedia , lookup
West Nile fever wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Marburg virus disease wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Henipavirus wikipedia , lookup
Antiviral drug wikipedia , lookup
Hepatitis C Update
Primary Care Guidance
Michael J. Tan, MD, FACP, FIDSA
Associate Professor of Internal Medicine
Northeast Ohio Medical University
Summa Health System
Akron, OH
Objectives
Review basic epidemiology of HCV
Understand testing and interpretation of HCV labs
Review new therapeutics
Primary source for recommendations is IDSA-AASLD,
accessible from www.hcvguidelines.org
2
What is HCV?
Non-A, Non-B
small, single stranded, enveloped RNA virus
Family: Flaviviridae
• Genera: Hepacivirus, HCV
• Genera: Flavivirus: Dengue, Yellow Fever, WNV
Targets hepatocytes
Blood-borne
2-3% of world’s population (130-170mil)
US <2%, Egypt > 10%
3
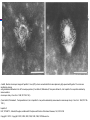
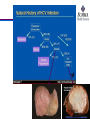
A and B, Electron microscopic images of hepatitis C virus (HCV) virions concentrated from human plasma by high-speed centrifugation. The virions are
identified by staining
with gold-labeled antibodies to the HCV envelope proteins. (From Kaito M, Watanabe S, Tsukiyama-Koham K, et al. Hepatitis C virus particle detected by
immunoelectron
microscopic study. J Gen Virol . 1994;75:1755-1760.)
(From Kaito M, Watanabe S, Tsukiyama-Koham K, et al. Hepatitis C virus particle detected by immunoelectron microscopic study. J Gen Virol . 1994;75:17551760.)
Hepatitis C
RAY, STUART C., Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, 154, 2157-2185
Copyright © 2010 Copyright © 2010, 2005, 2000, 1995, 1990, 1985, 1979 Elsevier Inc.
Hepatitis C Virus
• Primary target hepatocytes
• Genome encodes a polyprotein consists of 3011 amino
acids
– Structural (core, E1, E2)and Non-structural (regulatory NS2, NS3,
NS4A/B, NS5A/B) proteins
– Robust viral replication ~10 trillion particles/day
– RNA-dependent RNA polymerase-lacks proofreading and no DNA
intermediates
– Does not integrate into host genomes (vs. HIV)
HIV vs. HCV
• RNA virus-Retrovirus
• CD4, macrophages, dendritic
cells
• mutation rate
• Blood-borne, moderate sexual
transmission
• Fatal if untreated
• Treatable not curable
• Many drugs
• Life-long therapy
•
•
•
•
•
•
•
•
RNA virus-Flavivirus
Hepatocytes
mutation rate
Blood-borne, low sexual
transmission
Might be fatal (cirrhosis, HCC)
Both treatable AND potentially
curable
Few drugs (more to come)
Finite duration
Hepatitis C Virus
• Genotypes 1 to 6, multiple subtypes
• Geographical distribution differences around the world
• Genotypes important to stratify duration and dosing
– Genotype 1 harder and longer therapy
– Genotype 2,3 “easier” and shorter duration
– Less important with new and more potent agents
Geographic distribution of hepatitis C virus genotype. (Adapted from Fang JW, Chow V, Lau JY: Virology of hepatitis C virus. Clin Liver Dis 1997;1:493 - 514.)
(Adapted from Fang JW, Chow V, Lau JY: Virology of hepatitis C virus. Clin Liver Dis 1997;1:493 - 514.)
Viral Hepatitis
Cleveland Clinic,, Current Clinical Medicine, ${parentCitation.chapternum}, 539-551
Copyright © 2010 Copyright © 2009, 2010 by The Cleveland Clinic Foundation. Published by Saunders, a imprint of Elsevier Inc.
HCV Genotype (used to) matter
• Genotype 1 historically the most difficult type to treat
– Unfavorable characteristics
• Advanced fibrosis, especially cirrhosis
• African-American
• IL-28B CT or TT genotype
– Polymorphism encodes gene products for IFN-III
– Favorable response to CC genotype
• Baseline high viral load
• Prior non-responders
• New agents might relegate above characteristics,
except for cirrhosis
The Prevalence of Hepatitis C Virus Infection in
the United States, 1999 through 2002
•
•
•
•
•
•
•
NHANES data->15,000
Prevalence ~1.6%
~4.1 million + HCV Ab
~3.2 million with chronic HCV
Peak age 40-49 (4.3%)
Between age 20-59 (48.4%)
Risk factors: IVDU, >20 life-time sex partners, blood
transfusions >1992
Ann Intern Med. 2006;144(10):705-714. doi:10.7326/0003-4819-144-10-200605160-00004
From: The Prevalence of Hepatitis C Virus Infection in the United States, 1999 through 2002
Ann Intern Med. 2006;144(10):705-714. doi:10.7326/0003-4819-144-10-200605160-00004
Figure Legend:
Prevalence of antibodies to hepatitis C virus (HCV) by age group (A) and year of birth (B) in the Third National Health and Nutrition
Examination Survey (NHANES III, 1988–1994) and the current NHANES (1999–2002).The vertical bars represent 95% CIs.
Date of download:
4/2/2014
Copyright © American College of Physicians.
All rights reserved.
From: The Increasing Burden of Mortality From Viral Hepatitis in the United States Between 1999 and 2007
Ann Intern Med. 2012;156(4):271-278. doi:10.7326/0003-4819-156-4-201202210-00004
Figure Legend:
Annual age-adjusted mortality rates from hepatitis B and hepatitis C virus and HIV infections listed as causes of death in the United
States between 1999 and 2007.
Because a decedent can have multiple causes of death, a record listing more than 1 type of infection was counted for each type of
infection.
Date of download:
4/7/2014
Copyright © American College of Physicians.
All rights reserved.
• Traditional risk factors
Illegal drug injection (once or a
few times many years ago)
Clotting factors before 1987
Hemodialysis
Abnormal ALT
Prior transfusions or organ
transplantations before July 1992
HCW after needle sticks or
mucosal exposures with HCV +
blood
Children born to HCV + women
• Traditional risk factors
Illegal drug injection (once or a few times many
years ago)
Clotting factors before 1987
Hemodialysis
Abnormal ALT
Prior transfusions or organ transplantations
before July 1992
HCW after needle sticks or mucosal exposures
with HCV + blood
Children born to HCV + women
“Adults born during 1945-1965 should received onetime testing for HCV without prior ascertainment of HCV
risk”
Brief alcohol screening and intervention, if indicated
HIV-infected patients
Hepatitis C-not just an urban problem
Notes from the Field: Hepatitis C Virus
Infections Among Young Adults-Rural
Wisconsin 2010
• Increased HCV infections among persons
<30 age in 6 contiguous rural counties
2004-2008 ~8 cases/year
2009-2010 ~24 cases/year
Investigated all 25 patients-all HCV
Ab positive
17 patients-sharing hypodermic
needles, drug preparation
equipment or drug snorting
equipment
16 patients-injected/snorted/both
6 specimens with similar NS5B
regions
Subanalysis of quasispecies
not related
Staging
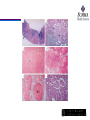
Histologic Stages of HCV Infection.
Lauer GM, Walker BD. N Engl J Med 2001;345:41-52.
Liver staging
Important to identify cirrhotic patients
Higher incidence of HCC
• Initiate surveillance, risk still present even patient is cured
Decreased response rate
Risk of liver decompensation (while on therapy)
Candidacy for response-guided therapy (1st generation protease
inhibitors: boceprevir and telaprevir)
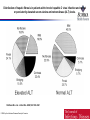
Distribution of hepatic fibrosis in patients with chronic hepatitis C virus infection and normal
or persistently elevated serum alanine aminotransferase (ALT) levels.
Shiftman M L et al. J Infect Dis. 2000;182:1595-1601
© 2000 by the Infectious Diseases Society of America
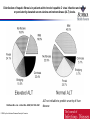
Distribution of hepatic fibrosis in patients with chronic hepatitis C virus infection and normal
or persistently elevated serum alanine aminotransferase (ALT) levels.
Shiftman M L et al. J Infect Dis. 2000;182:1595-1601
© 2000 by the Infectious Diseases Society of America
ALT not reliable to predict severity of liver
disease
Liver Biopsy
Still considers as gold standard
Might be replaced by serum markers
Values:
Best way to assess fibrosis
Assess other liver disease(s)
Definitive staging
Liver Biopsy
Cons:
Invasive
Relative more expensive than serum markers
Expertise in acquiring specimen and interpreting histopathology
• Adequacy of specimen
• Which classification is used (Metavir, Ishak, etc.)
Bleeding risk
Fig. 5
Source: Journal of Hepatology 2007; 47:598-607 (DOI:10.1016/j.jhep.2007.07.006 )
Copyright © 2007 Terms and Conditions
Liver Biopsy-size matters!!
>3 cm
1.5 cm
1 cm
22.4±4.9
10.3±2.2
6.4±1.2
Mild (≤6)
80 (49.7%)
97 (60.2%)
133 (86.6%)
Moderate (7-12)
62 (38.5%)
63 (39.1%)
28 (17.4%)
Severe (≥13)
19 (11.8%)
1 (0.6%)
0
Mild (1-2)
95 (59%)
110 (68.3%)
120 (80.1%)
Moderate (3-4)
48 (29.8%)
39 (24.2%)
24 (14.9%)
Severe (5-6)
18 (11.2%)
12 (7.4%)
8 (4.9%)
No. of portal
tracts
Complete
Grading
Staging
Colloredo et al. Journal of Hepatology 39 (2004):239-244
FibroSure
• Serum biomarkers as surrogate to test for fibrosis
• Non-invasive
• Biochemical markers: α2 macroglobulin, haptoglobin,
bilirubin, GGT, apolipoprotein A1 and ALT
• Coupled with patient’s age and gender and using a
proprietary artificial intelligence algorithm to generate a
score
FibroSure
Provides a numerical quantitative estimate of liver
fibrosis ranging 0.00 to 1.00-corresponds to Metavir
scroing F0-F4
F0=no fibrosis, F1= portal fibrosis, F2= bridging fibrosis with septa,
F3= bridging fibrosis with many septa, F4= cirrhosis
Provides a numerical quantitative estimate of
necroinflammatory activity from 0.00 to 1.00
corresponds to A0 to A3
A0= no activity, A1= minimal activity, A2= moderate activity, A3=
severe activity
FibroSure
Correlates fairly well with liver biopsy
Multiple studies indicated AUROC predictive value
between 0.70 to 0.80
Should not be used:
Hemolysis, acute hepatitis, autoimmune hepatitis, low haptoglobin,
genetic liver disease
Discordant liver biopsy vs. FibroSure
Biopsy size (<15 mm)
Hepatitis C More Background
Risks
About 10% spontaneous clearance
Non-cleared—risk of cirrhosis, liver failure, HCC
Previous treatments: Peg-Interferon + Ribavirin
Treatment up to 12mos
Sustained virologic response:
• Genotype 1: 70% (less with HIV)
• Genotype 2-3: 80-90% (less with HIV)
Anemia
Influenza-like illness
Depression
33
Treatment
Ghany et al. Hepatology, Vol. 49, April 2009
Therapy
For decades only two major classes of medications: interferon
and ribavirin
Standard IFN-1991; Pegylated IFN-early 2000’s
Response rate poor to fair at best (genotype 1 especially)
Moderately significant side-effects
Direct Acting Anti-virals (DAAs)-2011 revolutionized treatment
paradigm
Ribavirin
• Nucleoside purine analogs
• 1-β-D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide
• Broad spectrum of DNA and RNA viruses
– Exact mechanism on HCV not clear
• Major metabolite ribavirin-5’-triphosphate
– Erythrocytes lack necessary enzymes to degrade metabolite-leads to
accumulation of metabolites
– Exerts oxidative damage to cell membrane and increased extravascular hemolysis by RE system
Gilbert et al. AAC, Aug 1986, p.201-205
Evolution of HCV Therapy
McHutchison NEJM 1998; 339:1485-1492
Fried NEJM 2002; 347:975-982
Manns Lancet 2001; 358:958-965
Boceprevir
Telaprevir
Simeprevir
Asunaprevir
Sofosbuvir
Ledipasvir
Daclastavir
Organization of the hepatitis C virus (HCV) genome and polyprotein. ( A ) Organization of the HCV genome with nontranslated RNA segments shown as
lines and the open reading frame as a box; the region encoding the nonstructural proteins required for replication is shaded. ( B ) Functional organization
and processing of the viral polyprotein, showing approximate membrane topologies of the mature HCV proteins. Sites of cleavage by host cell and viral
proteases are indicated by triangles. (Redrawn from Lemon SM, Walker C, Alter MJ, et al. Hepatitis C virus. In: Knipe DM, Howley PM, eds. Fields’
Virology, 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007:1253.)
(Redrawn from Lemon SM, Walker C, Alter MJ, et al. Hepatitis C virus. In: Knipe DM, Howley PM, eds. Fields’ Virology, 5th ed. Philadelphia: Lippincott
Williams & Wilkins; 2007:1253.)
Viral Hepatitis C
Alter, Miriam J., Tropical Infectious Diseases: Principles, Pathogens and Practice, CHAPTER 65, 427-432
Anti-Hep C
4/2011, Recommended for approval
5/2011, Telaprevir, boceprevir-APPROVED!
Approval for recurrence/treatment
New Agents for HCV
Combination with PEG-IFN, RBV
Anemia biggest AE
Apparently better SVR than standard of care
Cost? Role? Efficacy in HIV?
Anti-Hep C-Boceprevir
N Eng J Med 2011;364:1195-206.
Boceprevir for untreated HCV Genotype 1
• Gp 1: pegIFN-RBV + Placebo 44wks
• Gp 2: peg IFN-RBV + boceprevir 24 wks
• Gp 3: peg IFN-RBV + boceprevir 44wks
SVR non-black: Gp 1 40% vs Gp 2 67% vs Gp 3 68%
SVR black: 23% vs 42% vs 53%
Anemia 13% controls vs 21% boceprevir
SVR rates improve with new DAAs
Adapted from: Poordad et al. NEJM 2011;364:1195; Jacobson et al. NEJM 20111:364:2405; Bacon et
al. NEMJ 2011;364:1207; Zeuzem et al. NEJM 2011;364:2417
Side-effects
Boceprevir
Fatigue
Anemia
Nausea
Dysgeusia
Telaprevir
Anemia
Rash
Fatigue
Pruritis
Nausea
Diarrhea
Vomiting
Hemorrhoids
Anorectal discomfort
Sofosbuvir (SOF, GS-7977)
HCV-specific uridine nucleotide NS5B polymerase inhibitor
(chain terminator)
Potent antiviral activity against
HCV genotypes 1–6
High barrier to resistance
Once-daily, oral, 400-mg tablet
Favorable clinical pharmacology
profile
No food effect
Renally cleared - limited potential for drug interactions
No CYP3A/4 metabolism
limited potential for drug interactions
Generally safe and well tolerated in clinical studies to date
(>3000 patients)
Gilead
Anti-HCV
Simeprevir (Janssen)
Oral 150mg q24 x12wk + Peg/Rib x24wk.
SVR 79-80%
Approval for naïve and treatment experienced Genotype 1
Once daily pill option
APPROVED
Sofosbuvir (Gilead)
Once daily nucleotide analogue
Approval in chronic HCV in adults with GT2, 3
• PEGIFN + Riba + sofosbuvir, Chronic HCV 1 and 4 treatment Naïve
• Riba + sofosbuvir, Chronic HCV 2, 3 (24wk)
• SVR in as little as 12 weeks Sup or non-inf to current treatments.
Sofosbuvir
Indications
Genotype 1 and 4: P/R/SOF x 12 weeks
• If INF ineligible: SOF + RBV x 24 weeks
Genotype 2: RBV + SOF x 12 weeks
Genotype 3: RBV + SOF x 24 weeks
Hepatocellular carcinoma awaiting transplant: RBV + SOF up to 48 weeks
HIV/HCV co-infection
Simeprevir (Olysio)
NS3/4A protease inhibitor
Combination therapy with PEG-IFN/RBV for genotype 1 only
Treatment naïve/prior relapses:
12 weeks then 12 more weeks with P/R
Partial/null responders and cirrhotics:
12 weeks then 36 more weeks with P/R
Simeprevir
Off label use-combination of sofosbuvir + simeprevir (sim-sof
combo)
As recommended by recent AASLD/IDSA joint guidelines for IFN intolerant
GT-1 patient
Significant: interferon free!
Data derived from ongoing study COSMOS
ledipasvir/sofosbovir (Harvoni)
Approved for genotype 1 in adults (too early to be
added to guidelines)
One tablet (90/400) PO q24h
Naïve with or without cirrhosis 12 wk
Experiencec without cirrhosis 12 wk
Experienced with cirrhosis 12 wk
Interferon sparing
AE:
Fatigue, Headache, nausea, diarrhea, insomnia
GT-1 96-99% SVR with minimal relapse
49
DAA
daclatasvir (Daklinza,)
paritaprevir/ritonovir/ombitasvir (daily) + dasabuvir
(twice/day) (Viekira Pak)
sofosbuvir (Sovaldi)
ledipasvir + sofosbuvir (Harvoni)
Simeprevir (Olysio)
When and in Whom to Initiate
Therapy?
All patients with chronic HCV except those with short
life expectancies that cannot be remediated by treating
HCV, by transplatation, or by other directed therapy.
Prioritization tables have been removed
Earlier treatment leads to augmented benefit of SVR
Treatment in setting of fibrosis or cirrhosis and reduce
decompensation
Recommendations-Treatment
Naive
Genotype 1
daclatasvir + sofosbuvir x12 weeks (no cirrhosis) (1a, 1b)
daclatasvir + sofosbuvir x24 weeks +/- ribavrin (cirrhosis) (1a, 1b)
ledipasvir + sofosbuvir x12 weeks (1a, 1b)
paritaprevir/ritonovir/ombitasvir (daily) + dasabuvir (twice/day) x12
weeks (no cirrhosis) or 24 weeks (cirrhosis) (1a)
paritaprevir/ritonovir/ombitasvir (daily) + dasabuvir (twice/day) x12
weeks (1b)
simeprevir + sofosbuvir x12 weeks (no cirrhosis) or +/- ribavirin
(cirrhosis) x 24 weeks (1b)
Not recommended:
• sofosbuvir + RBV x24wks
• PEG-IFN + RBV +/- Sofosbuvir, simeprevir, telaprevir, or boceprevir for 1248 weeks.
• Monotherapy with PEG-IFN, RBV, or DAA
52
www.hcvguidelines.com
Recommendations-Treatment
Naive
Genotype 2
daclatasvir + sofosbuvir x12 weeks (2) *Intolerant of ribavirin
sofosbuvir + RBV x12 wks
Extend treatment to 16 weeks with cirrhosis
Not recommended:
• PEG-IFN + RBV x24wks
• Monotherapy with PEG-IFN, RBV, or DAA
• Telaprevir, boceprevir, or ledipasvir containing regimens
Genotype 3
daclatasvir + sofosbuvir x12 weeks (no cirrhosis) or 24 weeks +/- RBV
x24 weeks (cirrhosis)
sofosbuvir + RBV + PEG-IFN x12wks (24 wks if no PEG-IFN)
Not recommended:
• PEG-IFN + RBV x24-48wks
• Monotherapy with PEG-IFN, RBV, or DAA
• Telaprevir, boceprevir, or ledipasvir containing regimens
Recommendations-Treatment
Naive
Genotype 4
ledipasvir/sofosbuvir x12 weeks
paritaprevir/ritonovir/ombitasvir + RBV x12 weeks
sofosbuvir + RBV x24 weeks
Alternative: sofosbuvir + RBV + PEG/IFN x12 weeks.
Not recommended:
• PEG-IFN + RBV +/- simeprevir x24-48wks
• Monotherapy with PEG-IFN, RBV, or DAA
• Telaprevir, boceprevir, containing regimens
Genotype 5/6
ledipasvir/sofosbuvir x12 weeks
Alternative: sofosbuvir + RBV + PEG-IFN x12wks
Not recommended:
• PEG-IFN + RBV +/- simeprevir x24-48wks
• Monotherapy with PEG-IFN, RBV, or DAA
• Telaprevir, boceprevir, or containing regimens
Recommendations-Retreatment
Genotype 1a, 1b, no cirrhosis, failed PEG-IFN-RBV
daclatasvir + sofosbuvir x12 weeks
ledipasvir + sofosbuvir x12 weeks
paritaprevir/ritonovir/ombitasvir (daily) + dasabuvir (twice/day) x12
weeks
simeprevir + sofosbuvir x12 weeks
1a, 1b, compensated cirrhosis, failed PEG-IFN-RBV
daclatasvir + sofosbuvir x24 weeks
ledipasvir + sofosbuvir x24 weeks (or with RBV for 12 weeks)
paritaprevir/ritonovir/ombitasvir (daily) + dasabuvir (twice/day) x12
weeks 1b, 24wks + RBV for 1a
simeprevir + sofosbuvir x24 weeks (1a Q80k (-)), 1b
55
www.hcvguidelines.com
Recommendations-Retreatment
Failed Sofosbuvir/RBV +/- PEG-IFN
ledipasvir/sofosbuvir + RBV x12 weeks (no cirrhosis)
ledipasvir/sofosbuvir + RBV x24 weeks (cirrhosis)
Failed tela/bocep/simep + PEG-IFN/RBV or sim/sof (NoNS5A),
geno 1
daclatasvir + sofos x12 weeks
Ledipasvir/sofos x12 weeks
Other combinations exist
Avoid in GT 1 for any protease failure:
Simepmrevir, sofosbuvir, telaprevir, or boceprevir with PEG-IFN/RBV or
PEG-IFN RBV alone
DAA or PEG-IFN, RBV monotherpay
PEG-IFN/RBV alone.
Any IFN-Free regimen containing Simeprevir or Paritaprevir
Recommendations-Retreatment
Genotype 2
Failed PEG-IFN, RBV: Sofosbuvir + RBV x16-24 wks
•
Add RBV and PEG-IFN for 12 weeks if eligible to receive PEG-IFN
Failed sofosbuvir and RBV:
• daclatasvir + sofosbuvir x24 wk +/- RBV
• Sofosbuvir + RBV + PEG-IFN x12 weeks.
Not recommended:
• PEG-IFN + RBV with or without telap or bocep
• Monotherapy with PEG-IFN, RBV, or DAA
• Ledipasvir/sofosbuvir
Genotype 3
daclatasvir + sofosbuvir x12 weeks (no cirrhosis) or + RBV x24 weeks (cirrhosis)
sofosbuvir + RBV + PEG-IFN x12 weeks
Prior sofos/RBV: daclatasvir + sofosbuvir + RBV x24 wks or sofos + RBV +
PEG-IFN x12 weeks.
Not recommended:
• PEG-IFN + RBV x24-48wks
• Monotherapy with PEG-IFN, RBV, or DAA
• Telaprevir, boceprevir, or ledipasvir containing regimens
Recommendations-Retreatment
Genotype 4
ledipasvir/sofosbuvir x12 weeks
paritaprevir/ritonovir/ombitasvir + RBV x12 weeks
Sofosbuvir x12 wks + RBV + PEG/IFN x12 weeks
sofosbuvir + RBV x24 weeks.
Not recommended:
• PEG-IFN + RBV +/- telaprevir or boceprevir
• Monotherapy with PEG-IFN, RBV, or DAA
Genotype 5/6
ledipasvir/sofosbuvir x12 weeks
Alternative: sofosbuvir + RBV + PEG-IFN x12wks
Not recommended:
• Monotherapy with PEG-IFN, RBV, or DAA
• Telaprevir, boceprevir, or containing regimens
Monitoring
Drug-Drug interactions
HCV Geno/Subtype, Qunatitative HCV
Within 12 weeks prior to start
CBC, INR, LFT, Calculated GFR
TSH (if IFN)
During therapy
CBC, Creat, GFR, LFT after4 weeks
TSH q12k weeks if IFN
Discontinue Tx for 10 fold rise in ALT at week 4.
• If less than 10 fold rise, check LFT wk 6 and 8
Quantitative @ 4 weeks, and 12 weeks after completion,
+/- 24 weeks after completion.
HCV and HIV
Still want to treat HIV fully
Follow guidelines for HCV
Watch for drug interactions with HAART
HCV
Decompensated disease has recommendations but
should be done with consultation with liver specialist
Post-liver transplant has recommendations
Specific recommendations for patients with impaired crt
cl <30
Acute infection?
HCV Ab and HCV RNA (viral load)
Pre-exposure prophy and post-exp prophy not recommended
F/U VL for clearance, no treatment for spontaneous clearance. (min
6 mos)
Monitoring
If HCV detectable @ wk 4, repeat at wk 6
If HCV increases 10 fold at week 6, stop therapy
If comes down at week 6 and 8, no recommendation
Check serum HCG
RBV not recommended in pregnancy
No SVR:
LFT, CBC, INR for disease progression
Surveillance for HCC q6mos
Endoscopic surveillance for varices if cirrhosis
Evaluate for retreatment as new alternatives become available.
SVR Achieved
No fibrosis (f/u as if no HCV)
Check Quant if ongoing risk of HCV or if unexplained
hepatic dysfunction
If F3 or F4, ultrasound 2x/year surveillance for HCC
Baseline endoscopy for varices if cirrhosis
Assess for other causes of liver disease in those who
develop persistently abnormal LFT after SVR
Not recommended to prospectively monitor for HCV
recurrence in those receiving immunosuppressive
therapy.
Conclusions
HCV is a huge problem
Cases on increase, especially with screening
Therapy is easier than it used to be
Cure is possible
Cost is an issue