* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ACID*BASE DISORDERS
Survey
Document related concepts
Transcript
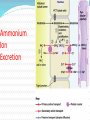
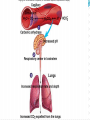
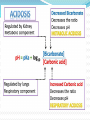
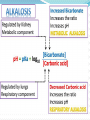
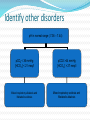
Normal Acid-Base Homeostasis Systemic arterial pH is maintained between 7.35 and 7.45 by extracellular and intracellular chemical buffering together with respiratory and renal regulatory mechanisms. The control of arterial CO2 tension (Paco2) by the central nervous system (CNS) and respiratory systems and the control of the plasma bicarbonate by the kidneys stabilize the arterial pH by excretion or retention of acid or alkali. Normal Values Arterial Venous 7.40 <7.35 HCO3 24 24 pCO2 40 >40 >70 <60 pH pO2 Effects of Metabolic acidosis Cardiovascular Impaired cardiac contractility Arteriolar dilation Venoconstriction Centralization of blood volume Increased pulmonary vascular resistance Decreased cardiac output Decreased systemic BP Decreased hepatorenal blood flow Decreased threshold for cardiac arrhythmias Attenuation of responsiveness to catecholamines METABOLIC ACIDOSIS METABOLIC EFFECTS Insulin resistance Inhibition of anaerobic glycolysis Reduction in ATP synthesis Hyperkalemia Protein degradation Bone demineralization (chronic) METABOLIC ACIDOSIS Neurologic Respiratory Inhibition of metabolism and Compensatory cell-volume regulation Obtundation and coma hyperventilation with possible respiratory muscle fatigue METABOLIC ALKALOSIS CARDIOVASCULAR Arteriolar constriction METABOLIC Stimulation of anaerobic glycolysis Reduced coronary blood flow Formation of organic acids Reduced anginal threshold Decreased oxyhemoglobin Decreased threshold for dissociation cardiac arrhythmias Decreased ionized calcium Hypokalemia Hypomagnesemia Hypophosphatemia METABOLIC ALKALOSIS NEUROLOGIC RESPIRATORY Tetany Compensatory Seizures Lethargy Delirium Stupor hypoventilation with hypercapnia and hypoxemia Acid–Base Balance Disturbances Disorders: 1. Circulating buffers Respiratory performance Renal function Cardiovascular conditions: 2. Heart failure Hypotension Conditions affecting the CNS: 3. Neural damage or disease that affects respiratory and cardiovascular reflexes Overview of Acid-Base Physiology Intracellular acid and base production Intravascular transport of acids or bases Elimination of acids and bases by kidneys and CO2 by the lungs Normal Serum Values Normal serum pH: 7.36 – 7.44 Normal serum [H+] : 40 mEq/L Normal serum [HCO3]: 24 mEq/L Normal serum pCO2 : 40 mmHg Acid–Base Balance Sources of Hydrogen Ions Most hydrogen ions originate from cellular metabolism Breakdown of phosphorus-containing proteins releases phosphoric acid into the ECF Anaerobic respiration of glucose produces lactic acid Fat metabolism yields organic acids and ketone bodies Transporting carbon dioxide as bicarbonate releases hydrogen ions Acid–Base Balance Hydrogen Ions (H+) Are gained At digestive tract Through cellular metabolic activities Are eliminated At kidneys and in urine At lungs Must be neutralized to avoid tissue damage Acids produced in normal metabolic activity Are temporarily neutralized by buffers in body fluids Acid–Base Balance Figure 27.7 Acid–Base Balance Major Buffers in Urine Glomerular filtration provides components of Carbonic acid–bicarbonate buffer system Phosphate buffer system Tubule cells of PCT Generate ammonia Carbonic acid– bicarbonate buffer system Phosphate buffer system Ammonium Ion Excretion Figure 26.14 Primary and Compensatory Changes in Acid-Base Disorders Disorder Metabolic acidosis Metabolic alkalosis Respiratory acidosis Respiratory alkalosis Primary Process HCO3 Compensation pCO2 HCO3 pCO2 pCO2 HCO3 pCO2 HCO3 MANAGEMENT General Guidelines in Treatment of Metabolic Acidosis 1. Identifying and correcting the specific underlying cause of the metabolic acidosis. 2. some types of metabolic acidosis will require HCO3- therapy and some will not. 3. The decision to use HC03- replacement should be weighed carefully, depending upon the severity of the acidemia (blood pH) and the type of acidosis. 4. If the pH falls below 7.10, emergency HC03- administration should be considered, regardless of the cause of the acidosis. This is especially important if there appears to be respiratory fatigue or developing hemodynamic instability 5. Never give HC03- without a determination of blood Ph 6. When the decision to give IV bicarbonate is made in the acute setting, calculate the amount of HCO3- required to increase the HC03- concentration to a specified value, often several mEq/L above the measured value. In general, assume that HC03- distributes in about 50% of Body weight (kg). HC03- deficit = 0.5 X Body weight (kg) X ([HCO3-(desired)]- [HC03(measured)]) 7. In severe acidosis (pH in the 7.10 range, HC03- <10mEq/L), the amount of HCO3required to increase the HCO3- concentration to the range 10-12mEq/L is initially calculated. For example, in a 70 kg patient, if the HC03- is 6 mEq/L, and it is desired to bring the HC03- to 10mEq/L: HC03- deficit = .5 X 70 X (10 mEq/L - 6 mEq/L) = 140mEqn Give this calculated amount slowly and remeasure pH, HC03- and PCO~after the HCO3- is given to assess the effect of therapy on the acid-base status. 8. The relationship between amount of HCO3- given and the increase in HCO3- is not linear: At mild levels of acidemia, 2 mEq/kg will increase the HC03- by roughly 4 mEqL. At severe levels of acidemia, 2 mEq/kg will only raise the HC03- by roughly 2 mEq/L. 9. In the case of an ongoing acidosis, repeated doses of HCO3- may be required until the underlying cause of the acidosis can be corrected. Treatment of L Lactic Acidosis Consider alkali therapy in cases of severe lactic acidosis when the pH falls below 7.10. When the underlying condition is corrected, however, lactate is converted to HC03-, and there may be an "overshoot alkalosis" during recovery. Treatment of Diabetic Ketoacidosis Diabetic ketoacidosis generally responds well to therapy with insulin, saline, and potassium. Because the circulating ketoanions will be converted to HC03- by the liver once insulin and fluids reverse ketosis, they represent "potential" HC03-. The majority of patients should not receive HC03- replacement for this reason. TreatmentofD-Lactic Acidosis Intravenous fluids and HC03-, but also requires oral antibiotics to eliminate the offending flora. Treatment of Alcoholic Acidosis Treatment consists of the administrationof dextrose-containing saline to reverse ketogenesis and correct any ECFV depletion. D5 0.9% saline with supplemental KC1 is usually appropriate for this purpose. HCO3- is not usually required because the ketones are converted to HC03-, once the ketosis is reversed and the ECFV normalized. In the case of alcoholic ketoacidosis with severe hypokalemia, the administration of Glucose should be postponed until potassium replacement is well underway Treatment of Distal (Type I) RTA Determine and correct the cause, if possible, and replace HC03and potassium. Distal RTA may require an amount of HC03- replacement that roughly equals the daily production of hydrogen ion (50-100 mEq/ day). Some of the HC03- should be given as KHC03 to correct potassium losses as long as there is no renal failure. Treatment of Type II (Proximal)RTA HC03- can be given as KHC03 (often as K-citrate) as long as there is no significant degree of renal failure. Mild to moderate hypokalemia is common in PRTA and is worsened by alkali therapy. Causes of Metabolic Alkalosis ECFV depletion--chloride depletion syndrome (saline-responsive) Vomiting/nasogastric suction Diuretic therapy Post hypercapnea Chronic diarrheal laxative abuse Severe potassium depletion from any cause (saline-resistant) Mineralocorticoid excess syndromes (saline-resistant) Primary hyperaldosteronism Cushing's syndrome Ectopic ACTH Secondary hyperaldosteronism Renovascular disease Malignant hypertension Congestive heart failure (with diuretic therapy) Cirrhosis (with diuretic therapy) Gitelman's syndrome (saline-resistant) Bartter's syndrome (saline-resistant) Metabolic alkalosis maintained by renal failure (saline generally contraindicated) Interpreting ABG - Check pH Step 1 – evaluate pH, narrow to 2 processes If the pH is < 7.36 Either metabolic acidosis or respiratory acidosis or both are present If the pH is >7.44 Either metabolic alkalosis or respiratory alkalosis or both are present Check pCO2 Step 2: evaluate pCO2 , narrow to 1 process For a pH < 7.36 If pCO2 < 40 - metabolic acidosis If pCO2 > 40 - respiratory acidosis For a pH > 7.44 If pCO2 < 40 - respiratory alkalosis If pCO2 > 40 - metabolic alkalosis Use proper compensation formula For metabolic acidosis: pCO2 = 1.5 [HCO3] + 8 (WINTER’S) For metabolic alkalosis: pCO2 = 0.9 [HCO3] + 16 Use proper compensation formula For respiratory acidosis: For every increase of 10 in pCO2: pH decreases by : 0.08 (acute) / 0.03 (chronic) HCO3 increases by: 1 meq/l (acute) / 3meq/l (chronic) Use proper compensation formula For respiratory alkalosis: For every decrease of 10 in pCO2: pH increases by : 0.08 (acute) / 0.03 (chronic) HCO3 decreases by: 2 meq/l (acute) / 4meq/l (chronic) Use proper compensation formula Simply choose the formula for the one disorder identified from step 1 and step 2. The purpose of using the appropriate formula is to discover if any other acid-base process is present ( to a lesser / greater degree ) Identify other disorders After applying chosen formula, compare the calculated (expected) value with the actual value If the measured pH, pCO2 , HCO3 doesn’t coincide with calculated value, then another acid-base disturbance is present Identify other disorders Step 4: For a metabolic acidosis: if actual pCO2 is higher than calculated – respiratory acidosis lower than calculated – respiratory alkalosis For a metabolic alkolosis: if actual pCO2 is higher by (>5mmHg) – respiratory acidosis lower by (>5mmHg) – respiratory alkolosis Identify other disorders Step 4: For a respiratory acidosis/ alkalosis: if pH or [HCO3] is higher – metabolic alkalosis if pH or [HCO3] is lower – metabolic acidosis What if pH is normal? [pCO2 & HCO3] abnormal An acidosis and alkalosis / to same degree Identify other disorders Possibilities: Metabolic alkalosis and respiratory acidosis Metabolic acidosis and respiratory alkalosis Metabolic acidosis and metabolic alkalosis Identify other disorders pH in normal range ( 7.36 – 7.44) pCO2 < 36 mmHg [HCO3 ] < 21 meq/l pCO2 >44 mmHg [HCO3 ] > 27 meq/l Mixed respiratory alkalosis and Metabolic acidosis Mixed respiratory acidosis and Metabolic alkalosis Anion gap Step 5: Evaluate anion gap [ Na – (Cl + HCO3)] If Elevated – indicate elevated gap - metabolic acidosis Check urine pH Step 6: Urine is normally acidic unless the serum is alkalemic. If urine is alkalotic (pH > 6.0) in face of an acidosis, a RTA or a UTI may be present Generate differential diagnosis Step 7: Identifying the primary pathology from analyzing differential diagnosis of various acid-base disturbances. Metabolic acidosis Metabolic acidosis HIGH ANION GAP (KUSSMALE) K – Ketosis (Diabetic, Alcoholic,) U – Uremia S – Salicylate poisoning S – Sepsis M – Methanol poisoning A – Alcohol (Ethanol Poisoning) L – Lactic acidosis E – Ethylene glycol NORMAL ANION-GAP RENAL RTA TYPE I RTA TYPE II RTA TYPE IV EARLY RENAL FAILURE NORMAL ANIONGAP EXTRARENAL DIARRHOEA GI-URETHRAL CONNECTIONS LOSS OF PANCREATIC AND BILIARY SECRETIONS CORRECTION PHASE OF DKA Metabolic alkalosis Metabolic alkalosis Saline (chloride) responsive Saline (chloride) unresponsive Diuretics Adenoma of colon Misc. (Bartter’s, penicillin K+ defi., bulimia) Posthypercapnia Emesis Nasogastric tube Alkali ingestion with decreased GFR 11 β hydroxylase deficiency Exogenous steroids Licorice ingestion Cushing’s syndrome Hyperaldosteronism Respiratory acidosis Respiratory acidosis Respiratory center depression: Sedative medications Brain stem lesions Central sleep apnoea, myxedema Neuromuscular failure: Myopathic and motor end plate dysfunction – polymyositis, hypokalemia, OPC Neuropathic – GBS, ALS, status epilepticus Decreased compliance Parenchymal – pulmonary fibrosis, ARDS Extra parenchymal – abdominal distension, severe kyphoscoliosis Increased airway resistance COPD, emphysema, severe asthma Obstructive sleep apnoea Increased dead space: Large pulmonary embolus Emphysema Respiratory alkalosis Hypoxia – pneumonia, pulmonary embolism, pulmonary edema, interstitial fibrosis Hyperdynamic states – pain, fever, sepsis, pregnancy, hyperthyroidism, hepatic failure, anxiety, mechanical hyprventilation CNS – CVA, tumor, infection, ICH, SAH Drugs – salicylates, catecholamines, progestrone, nicotine Pneumonics for pnuemonic lovers Metabolic Acidosis Anion Gap Metabolic Acidosis NonGap Acute Resp. Acidosis Metabolic Alkalosis Respiratory Alkalosis “CLEVERPD” “CHAMPS” “HARDUPS” “anything causing hypoventilation” “MUDPILERS” •Methanol •Hyperalimentation •CNS •Uremia •Acetazolamide •DKA/Alcoholic •Renal ketoacidosis •Paraldehyde •Isoniazid •Lactic acidosis •Ethanol •Renal failure/Rhabdo •Salicylates Tubular Acidosis •Diarrhea •Uretero-Pelvic shunt •Post-hypocapnia •Spironolactone depression •Contraction •Airway •Licorice obstruction •Endocrine •Pulmonary (Conn/Cushing edema /Bartters) •Pneumonia •Vomiting •Hemo/Pneumo •Excess alkali thorax •Refeeding •Neuromuscular •Posthypercapnia •Diuretics •CNS disease •Hypocapnia •Anxiety •Mech. Ventilation •Progesterone •Salicylates •Sepsis Interpretation – case 1 1) A surgeon refers a 22-year old man with a hernia to you, with history of renal stones. pH - 7.29 pCO2 – 32 mmHg [HCO3] – 15 meq/l Na+ - 138 meq/l K+ - 3.0 meq/l Cl- - 110 meq/l Urine pH – 6.0 Interpretation – case 1 Step 1 – pH < 7.36 So, pH - 7.29 Met. / resp. acidosis exist pCO2 – 32 Step 2 – pCO2 < 40 mmHg So, mmHg [HCO3] – 15 meq/l At least a Metabolic acidosis exists Step 3 – formula Expected pCO2 = 1.5[HCO3] + 8; i.e., = 30 Actual pCO2 = 32 mmHg Interpretation – case 1 Step 4 - any other process involved? Actual and expected pCO2 values match closely So, a metabolic acidosis, fully compensated exists Step 5 – evaluate anion gap Anion gap = 138 – (110 +15) = 13 A normal gap METABOLIC ACIDOSIS exists Case 2 A 40 year old lady with obesity and newly detected hypertension pH - 7.49 pCO2 – 45 mmHg [HCO3] – 33 meq/l Na+ - 142 meq/l K+ - 4.1 meq/l Cl- - 98 meq/l Urine pH – 6.5 Case 2 Step 1 – pH > 7.44 So, pH - 7.49 Met. / resp. alkalosis exist pCO2 – 45 Step 2 – pCO2 > 40 mmHg So, mmHg [HCO3] – 33 meq/l At least a Metabolic alkalosis exists Step 3 – formula Expected pCO2 = 0.9[HCO3] + 16; i.e., = 46 Actual pCO2 = 45 mmHg Case 2 Step 4 - any other process involved? pH - 7.49 Actual and expected pCO2 values appr. pCO2 – 45 match closely So, a metabolic alkalosis, fully compensated exists Step 5 – evaluate anion gap Anion gap = 142 – (98 +34) = 10 (normal) Step 6 – check urine pH = 6.5 ( in alkalemia) DD – cushings / hyperaldosteronism So, a simple Metabolic alkalosis exists mmHg [HCO3] – 33 meq/l Case 3 A 25 year old patient with fever, chest pain and breathlessness, was toxic and had a patch in his right middle lobe was treated for 3 days but respiratory rate was still high. pH - 7.47 pCO2 – 21 mmHg [HCO3] – 15 meq/l Na+ - 136 meq/l Cl- - 110 meq/l Urine pH – 6.5 Case 3 Step 1 – pH > 7.44 So, Met. / resp. alkalosis exist Step 2 – pCO2 < 40 mmHg. So, At least a respiratory alkalosis is present Step 3 – apply formula for chronic resp.alk. If we use pH : Expected pH – 7.40 + (2x0.03) = 7.46 Actual pH – 7.47 pH - 7.47 pCO2 – 21 mmHg [HCO3] – 15 meq/l Case 3 If we use [HCO3]: Expected [HCO3] – 24 - (2x4) = 16meq/l Actual [HCO3] – 15meq/l Step 4 - any other process involved? Actual and expected pH &[HCO3] values appr. match closely So, a chronic respiratory alkalosis, fully compensated exists. Step 5 – evaluate anion gap Anion gap = 136 – (110 +15) = 11 Step 6 – check urinepH =6.5(alkalemia) A simple chronic (fully compensated) resp. alk. exists pH - 7.47 pCO2 – 21 mmHg [HCO3] – 15 meq/l Case 4 If in the same patient – history is not known: pH - 7.47 pCO2 – 21 mmHg [HCO3] – 15 meq/l Na+ - 136 meq/l Cl- - 110 meq/l Urine pH – 6.5 With pH and pCO2 – respiratory alkalosis exists Case 4 Step 3 – apply formula for acute resp.alk. If we use pH : Expected pH – 7.40 + (2x0.08) = 7.56 Actual pH – 7.47 If we use [HCO3]: Expected [HCO3] – 24 - (2x2) = 20meq/l Actual [HCO3] – 15meq/l Step 4 - any other process involved? Actual pH &[HCO3] values are both LOWER than the expected values. So, which could lower both? Only a metabolic acidosis could lower both values So, a mixed resp. alkalosis and metabolic acidosis exists. pH - 7.47 pCO2 – 21 mmHg [HCO3] – 15 meq/l Case 5 A 20 year old man is brought to the emergency room by his sister, who tells you he took a bottle of pills pH - 7.35 pCO2 – 15 mmHg [HCO3] – 8 meq/l Na+ - 140 meq/l K+ - 3.5meq/l Cl- - 104meq/l Urine pH – 5.0 Case 5 Step 1 – pH < 7.36 So, pH - 7.35 Met. / resp. acidosis exist pCO2 – 15 Step 2 – pCO2 < 40 mmHg So, mmHg [HCO3] – 8 meq/l At least a Metabolic acidosis exists Step 3 – formula Expected pCO2 = 1.5[HCO3] + 8; i.e., = 20 Actual pCO2 = 15 mmHg Case 5 Step 4 - any other process involved? Actual pCO2 value is less than expected Only process that could decrease the pCO2 value beyond predicted is RESPIRATORY ALKALOSIS So, a mixed metabolic acidosis & resp. alk. exists Step 5 – evaluate anion gap Anion gap = 140 - (104 + 8) = 28 (elevated) Urine pH = 5.0 (fits to acidemia) A mixed elevated gap Metabolic acidosis and Respiratory alkalosis exists DD – only sepsis/salicylate overdose cause both So, given the history salicyalate overdose is possibile Case 6 A 57 year old patient with long history of smoking presents without distress and with h/o BOE, ABG shows: pH - 7.35 pCO2 – 50 mmHg [HCO3] – 27 meq/l Na+ - 143 meq/l Cl- - 105 meq/l Urine pH – 5.0 Case 6 Step 1 – pH < 7.36 So, Met. / resp. acidosis present Step 2 – pCO2 > 40 mmHg So, At least a respiratory acidosis is present Step 3 – apply formula for respiratory acidosis If we use pH : use chronic formula Expected pH – 7.40 - (1x0.03) = 7.37 Actual pH – 7.35 pH - 7.35 pCO2 – 50 mmHg [HCO3] – 27 meq/l Case 6 If we use [HCO3]: Expected [HCO3] – 24 + (1x3) = 27 meq/l Actual [HCO3] – 27meq/l Step 4 - any other process involved? Actual and expected pH &[HCO3] values appr. match closely So, a pure chronic respiratory acidosis, fully compensated exists. Step 5 – evaluate anion gap Anion gap = 143 – (105 +28) = 10(normal) Step 6 – check urine pH = 5.0 ( fit for acidemia) A simple chronic fully compensated resp. acid. exists pH - 7.35 pCO2 – 50 mmHg [HCO3] – 27 meq/l Case 6 The same patient presents to emergency room 1 month later in respiratory distress. He has wheezing and his RR – 33 B/min pH - 7.29 pCO2 – 61 mmHg [HCO3] – 28 meq/l Na+ - 142 meq/l Cl- - 100 meq/l Urine pH – 5.0 Case 6 Step 1 – pH < 7.36 So, Met. / resp. acidosis present Step 2 – pCO2 > 40 mmHg So, At least a respiratory acidosis is present Step 3 – apply formula for respiratory acidosis, both acute and chronic If we use pH : chronic Expected pH – 7.40 - (2x0.03) = 7.34 Actual pH – 7.29 pH - 7.29 pCO2 – 61 mmHg [HCO3] – 28 meq/l Case 6 If we use [HCO3]: chronic pH - 7.29 Expected [HCO3] – 24 + (2x3) = 30 meq/l pCO2 – 61 Actual [HCO3] – 28 meq/l mmHg Actual pH &[HCO3] values are lower than [HCO3] – 28 expected: meq/l Possibilities: 1) Mixed resp. acid. / small met. acid. 2) Resp. acid. – partially compensated (acute – on –chronic / mixed acute & chronic resp. acid.) Case 6 If we use pH : acute Expected pH – 7.40 - (2x0.08) = 7.24 Actual pH – 7.29 If we use [HCO3]: acute Expected [HCO3] – 24 + (2x1) = 26 meq/l Actual [HCO3] – 28meq/l Actual pH &[HCO3] values are higher than expected: Possibilities: 1) a mixed acute resp.acid./small met. Alk. 2) a resp. acid. that is partially compensated. pH - 7.29 pCO2 – 61 mmHg [HCO3] – 28 meq/l Case 6 Step 4 – identify other processes Summarising step 3 : 1)A mixed chonic resp.acid./small met. Acid. 2)An acute resp. acid./ small met. Alk. 3) A resp.acid. Not fully compensated In our patient we consider the third possibility since patient is having acute exacerbation of COPD So, a mixed acute and chronic resp. acidosis. Case 7 A 45 year old diabetic patient presents with obtundation pH - 7.01 pCO2 – 80 mmHg [HCO3] – 20 meq/l Na+ - 140 meq/l K+ - 5.5 meq/l Cl- - 97 meq/l Urine pH – 5.0 Case 7 Step 1 – pH < 7.36 So, Met. / resp. acidosis exist Step 2 – pCO2 > 40 mmHg So, At least a respiratory acidosis exists Step 3 – formula – acute resp. acid. If we use pH : acute Expected pH – 7.40 - (4x0.08) = 7.08 Actual pH – 7.01 pH - 7.01 pCO2 – 80 mmHg [HCO3] – 20 meq/l Case 7 If we use [HCO3]: acute pH - 7.01 Expected [HCO3] – 24 + (4x1) = 28 pCO2 – 80 meq/l Actual [HCO3] – 20 meq/l Actual and expected pH & [HCO3] values are lower than expected: Since [HCO3] value is lower as against an increase a metabolic acidosis coexists mmHg [HCO3] – 20 meq/l Case 7 Step 5 – evaluate anion gap Anion gap = 140 – (97 +20) = 23(elevated) Step 6 – check urine pH = 5.0 ( fit for acidemia) DD – predominant respiratory acidosis and an elevated gap metabolic acidosis (DKA) Case 8 A 78 year old patient has been vomiting for several days, developed fever and breathlessnessover few hours. Her RR – 35b/min and has consolidation over Rt. Base. pH - 7.69 pCO2 – 20 mmHg [HCO3] – 25 meq/l Na+ - 138 meq/l Cl- - 97 meq/l Urine pH – 8.0 Case 8 Step 1 – pH > 7.44 So, Met. / resp. alkalosis exist Step 2 – pCO2 < 40 mmHg So, At least a respiratory alkalosis exists Step 3 – formula – acute resp. alkalosis If we use pH : Expected pH – 7.40 + (2x0.08) = 7.56 Actual pH – 7.69 pH - 7.69 pCO2 – 20 mmHg [HCO3] – 25 meq/l Case 8 If we use [HCO3]: Expected [HCO3] – 24 - (2x2) = 20meq/l Actual [HCO3] – 25meq/l Step 4 - any other process involved? Actual pH &[HCO3] values are both higher than the expected values. So, which could increase both? Only a metabolic alkalosis could elevate both values Step 5 – evaluate anion gap Anion gap = 138 – (97 +28) = 13(normal) Step 6 – check urine pH = 8.0 ( fit for alkalemia) A mixed acute resp. alk. & met. Alk. pH - 7.69 pCO2 – 20 mmHg [HCO3] – 25 meq/l Case 9 A 27 year old diabetic patient with 1hr of shortness of breath. He was nauseated and has had increased urination over the past 2 days. So, he didn’t take insulin and was bedridden for past 2 days. He has family history of hypercoagulable disorder. Case 9 pH - 7.40 pCO2 – 20 mmHg [HCO3] – 12 meq/l Na+ - 136 meq/l Cl- - 102 meq/l Urine pH – 5.0, ketones + Glucose – 650 mg/dl For pH in normal range pH in normal range ( 7.36 – 7.44) pCO2 < 36 mmHg [HCO3 ] < 21 meq/l pCO2 >44 mmHg [HCO3 ] > 27 meq/l Mixed respiratory alkalosis and Metabolic acidosis Mixed respiratory acidosis and Metabolic alkalosis Case 9 Step 1 – pH is 7.40 (normal) pH - 7.40 pCO2 and [HCO3] are abnormal, So pCO2 – 20 A mixed acid-base disturbance is present mmHg [HCO3] – 12 meq/l At least one acidosis and one alkalosis present Alkalosis is exactly balancing acidosis Case 9 Step 2 pH - 7.40 pCO2 < 40 mmHg and [HCO3] < 25 meq/l pCO – 20 2 Respiratory alkalosis & metabolic acidosis mmHg is the process that can cause this [HCO3] – 12 Step 3 – check with formula for both meq/l Metabolic acidosis: Expected pCO2 = 1.5[HCO3] + 8; i.e., = 26 Actual pCO2 = 20 mmHg Actual is lower than expected value, So a respiratory alkalosis is lowering pCO2 Case 9 For respiratory alkalosis: If we use pH : Expected pH – 7.40 + (2x0.08) = 7.56 Actual pH – 7.40 If we use [HCO3]: Expected [HCO3] – 24 - (2x2) = 20 meq/l Actual [HCO3] – 12 meq/l Step 4 - any other process involved? Actual pH & [HCO3] values are lower than expected. So, a metabolic acidosis exists. pH - 7.40 pCO2 – 20 mmHg [HCO3] – 12 meq/l Case 9 Step 5: evaluate anion gap Anion gap = 136 – (102+12) = 24 An elevated gap metabolic acidosis is present Step 6 : check the urine pH Urine pH = 5.0 (fit for acidemia) DD – 1) DKA – elevated gap metabolic acidosis 2) an acute respiratory alkalosis - ?PE So, both equally balances to maintain pH. Thank you