* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Intervention Technique using Transvenous Patent Ductus Arteriosus
History of invasive and interventional cardiology wikipedia , lookup
Coronary artery disease wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Artificial heart valve wikipedia , lookup
Aortic stenosis wikipedia , lookup
Cardiac surgery wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Echocardiography wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
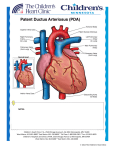
Original Article Intervention Technique using Transvenous Patent Ductus Arteriosus (PDA) Occluder in a Dog Chollada Buranakarl1* Anusak Kijtawornrat1 Wasan Udayachalerm2 Sirilak Disatian Surachetpong3 Saikaew Sutayatram1 Pasakorn Briksawan4 Suwicha Chuthathep4 Rampaipat Tungjitpeanpong5 Nardtiwa Chaivoravitsakul5 Abstract The first case interventional technique of Patent Ductus Arterious (PDA) correction was conducted in a 2 years old Golden Retriever. The dog was presented with continuous murmur with a marked left-side heart enlargement, insufficiency of pulmonic, mitral, and aortic valves. The definitive diagnosis for PDA was performed by echocardiography. The dog received PDA correction with interventional PDA occluding device consisting of nitinol wire mesh under fluoroscope at the Small Animal Teaching Hospital, Faculty of Veterinary Science, Chulalongkorn University with collaboration by medical expertise from Faculty of Medicine, Chulalongkorn University. The procedure was successful as measured by complete occlusion of PDA and no major complication. At one month after correction, the dog was more healthy and energetic while echocardiography showed a reduction of left ventricular dimension and improved valvular insufficiency. At six months after correction, the dog had smaller heart size with improved cardiac function. Keywords: dog, PDA occluder, intervention technique 1Department of Physiology, 3Department of Medicine, 4Department of Surgery, 5Small Animal Teaching Hospital, Faculty of Veterinary Science, Chulalongkorn Univerisity 2 Department of Physiology, Faculty of Medicine, Chulalongkorn Univerisity Pathumwan, Bangkok, 10330, Thailand. *Correspondence: [email protected] Thai J Vet Med. 2015. 45(4): 593-602. 594 Buranakarl C. et al. / Thai J Vet Med. 2015. 45(4): 593-602. Introduction Patent ductus arteriosus (PDA) is a congenital heart disease that found in both humans and animals in which the vessel connected between aorta and pulmonary artery is not vanished after birth. The changes in smooth muscle wall made the vessel maintained its structure without constriction and fibrosis. Thus, the oxygenated blood from left ventricle can be passing through the main pulmonary artery during ejection called “left to right shunt”. Since the workload of the left heart increases to maintain sufficient cardiac output, the left ventricular wall becomes hypertrophy and dilated. The animals may show signs of exercise intolerance when they are puppy and the disease may become more progressive up until the left heart failure developed along with pulmonary edema. The correction has to be performed as soon as possible after PDA diagnosis to prevent animal from left heart failure and death. The curative treatment by thoracotomy to ligate the duct has been performed with success. However, the procedure is highly invasive with high incidence rate of postoperative complications. At present, the interventional technique has been introduced into human and veterinary medicine. The transvenous catheterization to put the device into the area of ductus for blood flow occlusion has been performed in human worldwide. In dogs, the first transvascular occlusion of PDA was reported by Miller and colleagues (1994). Many devices including coil and mesh have been used to occlude PDA with varying indications and outcomes (Singh et al., 2012). This is the first case report in Thailand to correct PDA using interventional PDA occluding device with excellent outcome. History A two years old spayed female Golden Retriever weighting 31 kg was present to Small Animal Teaching Hospital, Chulalongkorn University, Bangkok, Thailand, with history of heart murmurs and abnormal echocardiography assessed by local Veterinarians without obvious cardiovascular signs. Figure 1 No exercise intolerance or syncope was found while she was exercised regularly. Physical examination revealed high heart rate, normal lung sound, and continuous heart murmur grade IV/VI with point of maximal intensity (PMI) at base of the heart on the left side. The patent ductus arteriosus was suspected while further tests were performed to rule out other congenital cardiac diseases. Diagnosis Routine blood collection and blood gas were performed and the results were within normal limits. The thoracic radiograph was performed and showed left heart enlargement on both ventrodorsal and lateral views with vertebral heart score of 13.5. The pulmonary congestion and mild edema were also noticed (figure 1). The electrocardiography was recorded and showed heart rate of 91 beats per minute of normal sinus arrhythmia with tall R-wave (4.4 mV) (figure 2). For the definite diagnosis of PDA, the echocardiography was performed by the experienced sonographer using 2-4 MHz phase array transducer and ultrasound machine (Eko7 Cardiovascular Ultrasound System, Samsung Medison). The examination was conducted without sedation when the dog lying down in right and left lateral recumbence. Enlarged left atrium, ventricle and pulmonary artery were seen on two-dimensional echocardiography (figure 3). Doppler on twodimensional echocardiography revealed functional mitral, aortic and pulmonic valves insufficiency. Continuous turbulent flow was observed within pulmonary artery. Spectral Doppler showed upright saw tooth flow pattern with velocity 4.28 m/s and pressure gradient at least 73.33 mmHg (figure 4). Increased aortic flow velocity was observed. On left cranial transverse view, the ductus with minimal ductal diameter 6.8 mm and ampulla diameter 15.6 mm was measured. The cardiac dimensions and functions were evaluated as shown in table 1. The dog was scheduled to have transcatheter intervention by PDA occluder two weeks later. Thoracic radiograph of ventrodorsal (left) and lateral (right) views showing the enlargement of the left heart with pulmonary congestion and mild pulmonary edema. Buranakarl C. et al. / Thai J Vet Med. 2015. 45(4): 593-602. 595 Figure 2 ECG recorded form PDA dog showed high voltage of QRS complexes. Figure 3 Two-dimensional echocardiography on right parasternal view showed left atrial and ventricular enlargement (A). Color Doppler of two dimensional echocardiography on right parasternal and transverse views showed mild mitral valve regurgitation (B) mild aortic valve insufficiency (C) and mild pulmonic valve insufficiency (D). LA=left atrium; LV=left ventricle; MV=mitral valve; AO=aorta; PA=pulmonary artery; RV=right ventricle. 596 Figure 4 Buranakarl C. et al. / Thai J Vet Med. 2015. 45(4): 593-602. Velocity of blood flow and flow pattern through ductus assessed by continuous wave Doppler echocardiography on right transverse view (A). Ductus oriented from pulmonary artery to aorta on modified left cranial transverse view of two dimensional echocardiography (B). Color Doppler on two dimensional echocardiography demonstrating flow from ductus to pulmonary artery (C). AO=aorta; PA= pulmonary artery Treatment Procedure for Transcatheter Patent Ductus Arteriosus (PDA) Occlusion: On the operation day, the dog was premedicated with midazolam (0.3 mg/kg, iv) and fentanyl (5 µg/kg, iv) and anesthetized with etomidate (1 mg/kg, iv). Dog was placed on right lateral recumbency. After orotracheal intubation, anesthesia was maintained by isoflurane 1.5-2% in 100% oxygen. Morphine sulfate (0.5 mg/kg, im) was also given for analgesia. Prophylactic antibiotic (cephazolin 25 mg/kg, iv) and intravenous fluid (Lactate Ringer Solution 10 mL/kg/hr) were administered via cephalic vein. The right inguinal region was prepared for surgery by aseptic technique. An incision approximately 2-3 cm was made over the right femoral triangle to visualize femoral artery and vein. The Seldinger technique was used to place vascular access sheets (figure 5) into femoral artery (5 Fr) and vein (6 Fr). Basically, the intravenous catheter was placed into the vessel followed by advancing the guidewire through the needle. After that the needle was removed and the vessel dilator together with vascular access sheet was advanced over the guidewire into the vessel. Then the guidewire and the vessel dilator were removed and the sheet was flushed with heparinized saline. A heparin (3 iu/kg, iv) was given to the dog before the beginning of cardiac catheterization. Ductal Angiography: In order to determine the PDA occluder size, the ductal ostium was visualized by cardiac catheterization and angiography technique. In this case, a pigtail angiographic catheter (Cook Medical, Thailand; 4 Fr) was inserted into femoral artery via the vascular access sheet. Under fluoroscopic guidance (Phillips® BV Endura), the tip of the catheter was positioned near the ostium (cranial or caudal) of the ductal ampulla. Angiography was performed with a rapid rate of administration (15 ml/ s; dependent on catheter flow limitations) of non-ionic iodinated contrast medium (Iohexol, Omnipaque® 300 mgI/ml, GE Healthcare, Shanghai, China) at a dosage of approximately 1 ml per kg bodyweight through the angiographic catheter in aorta. The contrast material was injected via a dual-syringe power injector system (Medtron® AG, Accutron CT-D, Germany) to identify the position of the ductal ampulla. The size of the PDA in this dog could not be seen clearly. Therefore, the size of the PDA occluder was selected based on the echocardiographic examination. In this dog, the cocoon duct occluder (size 12/14/20 mm, Vascular Innovations, USA) was selected. The number 12 is the Buranakarl C. et al. / Thai J Vet Med. 2015. 45(4): 593-602. device diameter at pulmonary artery, the number 14 is the device diameter at descending aorta, and the number 20 is the retention diameter. PDA duct Occluder Deployment : After determination of the PDA size, a 5 Fr 45 degree angled end-hole catheter (Cook Medical, Thailand) was advanced into the femoral vein and directed to the PDA. A 0.035” straight, floppy tip exchange wire is directed through this catheter lumen and advanced across the PDA into the aorta. The length of the wire is 200 cm which is approximately twice the length of the catheter. Then the wire was maintained within the aorta while the end-hole catheter was withdrawn. The delivery sheath with its dilator in place (8 Fr, Figure 5) was directed over the exchange wire and advanced across the ductus into the aorta. The dilator was carefully withdrawn into the sheath to minimize potential damage while the sheath is passing through the duct. The dilator and exchange wire were completely removed when the sheath tip was inside the aorta. Then a hemostasis valve (Figure 5) was applied on the sheath end. The PDA duct occluder device (Figure 6) was loaded under sterile saline to prevent air emboli during deployment. Then the hemostasis valve was removed and the loader tip was inserted on the sheath and advancing the device into the lumen. The loader was withdrawn from the sheath to prevent hemorrhage and the duct occluder device was 597 advanced through the sheath lumen by pushing on the delivery cable (figure 6) while the sheath tip was still remained in the aorta. After the PDA duct occluder device was exteriorized within aorta, the sheath and device were withdrawn together until the disc stayed within the ampulla with distal disc engaged with the ostium. Gentle retraction was applied on the delivery cable to keep the device in contact with the ostium. Unfortunately, the PDA duct occluder device was too small; therefore, the device was pulled back into the pulmonary artery. A new PDA duct occluder device (size 16/18/24 mm) was then used and the process of occluder deployment was restarted again. After the new occluder was in proper location, a contrast medium (20 mL) was administered by hand injection to document device position. Once the device is stable with its casted shape, the manufacturer-supplied vice pin was attached to the delivery cable and rotated in a counterclockwise direction until the device was released from microscrew. Then the cable and the sheath were withdrawn together. After that the vascular access sheath was removed from both femoral artery and vein and a pressure was applied on the skin over the vessels to stop bleeding. A combat gauze trauma pad was used to stop bleeding before closing the incision site with routine manner. Analgesic (morphine sulphate, 0.5 mg/kg, iv) and another dose of cephazolin were given and the patient was admitted in the hospital for monitoring. Figure 5 Shows catheters for Seldinger technique and delivery sheath with its dilator. Figure 6 Shows PDA duct occluder device attached to the delivery cable. The loader catheter and hemostasis valve are also included in this picture. 598 Buranakarl C. et al. / Thai J Vet Med. 2015. 45(4): 593-602. Immediately after PDA was corrected, the machinery murmur was disappeared and HR was suddenly dropped down to approximately 40-60 bpm with normal systolic blood pressure. Post-operative medications included antibiotic (amoxicillinclavulanic acid) and analgesic drugs (tramadol). No major complication was found with minimal swelling at the surgical site. Three days after device occlusion, echocardiography revealed the left atrium and ventricle were decreased in size. However, the mild functional mitral and aortic insufficiency as well as Figure 7 trivial functional pulmonic insufficiency was observed. Two-dimensional echocardiogram revealed the device in ductus on modified right parasternal short axis view and modified left cranial transverse view (figure 7). No turbulent flow was detected in the pulmonary artery suggesting the PDA was occluded completely. The pulmonary artery flow could be measured with maximal velocity 1.14 m/s with pressure gradient 5.24 mmHg. The dog was discharged on day 3 after surgery. Two-dimensional echocardiography demonstrating device in ductus on right parasternal short axis view (A) and modified left cranial transverse view (B). PA = pulmonary artery; PV = pulmonic valve. Color flow Doppler and spectral Doppler echocardiography demonstrating flow in pulmonary artery with velocity 1.14 m/s and pressure gradient 5.24 mmHg after device occlusion on modified right parasternal short axis view (C). Thirty days after device occlusion, the size of aortic root was decreased. Mild functional mitral, aortic and trivial pulmonic insufficiency was seen. Fractional shortening was still lower than normal limit. However, left ventricular chamber dimension during systole and diastole were decreased. One hundred and eighty day after device occlusion, echocardiographic examination revealed the decrease of jet sizes in all valves (mitral, aortic and pulmonic valves). The FS and EF were higher than one month after occlusion. The echocardiographic values before and after correction of PDA were shown in Table 1. Discussion Patent ductus arteriosus is a congenital heart disease commonly found in many small and large breed dogs (Buchanon, 1992). The abnormal development of smooth muscle of the duct leads to the post-partum closure failure of the vessel that connecting between aorta and pulmonary artery. In case of PDA, structures involved in the shunt pathway including descending aorta, pulmonary artery, left atrium and left ventricle are usually enlarged. The diagnosis of PDA is based upon the echocardiography. The left cranial transverse view is a better view to reveal the ductus compared to the right transverse view (Schneider et al, 2007). The upward saw toothed flow profile is a pathognomonic flow pattern of PDA. This pattern occurs secondary to the difference of pressure between aorta and pulmonary artery during systole and diastole. Pressure gradient between aorta and pulmonary artery should be closed to 100 mmHg. As in this dog, the pressure gradient was Buranakarl C. et al. / Thai J Vet Med. 2015. 45(4): 593-602. around 70 mmHg. A high aortic flow velocity suggested a significant ductal shunting in this dog. Measurements of ductal size can be performed using echocardiogram. Size of ductus measured by echocardiogram has been suggested to be closed to a true size measured by angiogram (Saunders et al, 2007). This dog has a larger ampulla diameter compared to a minimal ductal diameter confirming a tapering type of ductus, which is fitted for catheter based occlusion of PDA (Schneider et al, 2007). The pulmonary artery usually dilates at the level of the pulmonic valve and the right and left main pulmonary artery branches (Boon, 2011). Flow from Table 1 599 ductus is up toward the pulmonary valve which may cause pulmonic valve prolapse or cusp annulus dilatation causing insufficiency (Boon et al, 2011) as seen in this dog with mild pulmonic insufficiency. The dog in this report also had a decrease in % fractional shortening and % ejection fraction similar to results of Hamabe and colleagues (2015). This is because systolic wall stress is not elevated due to the far away shunt and the left ventricular free wall and interventricular septum are inadequate hypertrophy during systole (Van Israël et al, 2002). Echocardiographic value before and after device occlusion groups Parameter Before 3 days after 30 days after 180 days after reference* Ao diameter (mm) 25.4 27.2 22.4 23.5 17.0-30.7 LA diameter (mm) 41.4 33.4 39.8 41.4 21.5-36.8 LA/Ao ratio 1.63 1.23 1.78 1.76 0.8-1.1 IVSd (mm) 11.3 11.8 11.5 11.6 10.3-11.5 LVd (mm) 67.2 61.9 52.7 49.4 32.3-43.9 LVPWd (mm) 10.3 12.3 11.5 10.3 8.3-9.3 IVSs 11.8 15.8 15.8 12.7 15.7-17.0 LVs 49.8 54.4 44.7 39 19.0-29.2 LVPWs 14.6 12.7 14.8 13.6 13.4-14.7 %Fractional shortening 25.87 12.06 15.18 20.95 33-46 %Ejection fraction 49.67 25.51 31.87 42.50 50-60 AV max (m/s) 2.84 1.24 1.24 1.18 <2 PV max (m/s) n/a 1.14 1.44 0.96 <1.6 *Boon (2011) Ao = aorta; LA = left atrium; IVSd = thickness of interventricular septum during diastole; IVSs = thickness of interventricular septum during diastole; LVd = left ventricular dimension during diastole; LVs = left ventricular dimension during systole; LVPWd = thickness of left ventricular free wall during diastole; LVPWs = thickness of left ventricular free wall during systole; AV max = aortic flow; PV max = pulmonary artery flow The correction of PDA showed positive effect on survival and reverse cardiac remodeling in the longterm follow up (Saunders et al., 2014). The severity of cardiovascular signs may depend on the ostium diameter and other complicated cardiac disease. The left to right shunt from PDA can induce the left side congestive heart failure with systolic dysfunction if PDA correction is not performed early in life. Stauthammer (2015) suggested that the dogs with congestive heart failure have an excellent outcome if PDA occlusion is performed before one year of age. Moreover, after PDA closure in dogs, the survival rate was negatively correlated with clinical signs, concurrent congenital heart disease and severe mitral regurgitation (Saunders et al., 2014). Treatment of choice includes a conventional surgical ligation. However, it had higher risks and complications. The mortality and complication rates after this surgical procedure may be high. Therefore, the interventional methods that are less invasive with high success have been introduced in both human and animals. At present, both the occlusion devices and procedures have been developed to introduce occluder via transvenous or transarterial intervention. Introductions of device occluder using vascular catheters have been used and published (Snaps et al., 1995, Grifka et al., 1996, Schneider et al., 1996, Fellows et al., 1998, Fox et al., 1998, Glaus et al., 1999, Glaus et al., 2002). Among these catheter techniques, the transarterial coil-occlusion is a non-invasive and safe, but it is suitable for dogs with small diameter of ducts. This technique also showed several complications included incomplete ductal closer, ductal reopening, death, transient hemoglobinuria, hemorrhage, 600 pulmonary hypertension, and embolization (Campbell et al., 2006). The most excellent outcome in dog is transarterial Amplatzer Canine Duct Occluder (ACDO) which composed of multilayer nitinol wire mesh, compared with other interventions [i.e., transarterial Gianturco or Reye Flipper Detachable Embolization (Flipper) coil, transarterial Amplatzer Vascular Plug, and transvenous Flipper coil] (Singh et al., 2012). However, in the present case, the transvenous occluder was chosen. In term of choosing the device size, Glausand coworkers (2002) suggested that the PDA occluder device which was similar to the present study should be 2 size larger than the PDA measured by contrast injection. In the present study although the 12 mm occluder (at pulmonary ostium) was chosen, the first attempt was failed which may be due to an inaccurate measurement of the ductal diameter using echocardiography. The angiographic measurement of the PDA in this dog could not be visualized due to position of the tip of catheter or limitation of the injector to overcome arterial pressure. One report suggested that the size selection of the device by angiography is dominated and could not be substituted by echocardiography (Nguyenba and Tobias, 2007). The second intervention was performed with success when occluder with 4 mm larger than the first device at pulmonary ostium was introduced. Immediately after PDA closer, heart rate was dropped to a normal value without murmur sound suggesting the occlusion may be completed. The dog was recovered quickly without complication. The surgical size was swelling due to compression bandage without vascular suturing. Improvement of hemodynamic indexes from echocardiography occurred within 24 hour after occlusion. After PDA closure, systolic function is commonly poorer. Percent fractional shortening in this dog was declined to 12.06%. A decrease of fractional shortening may occur secondarily to a decrease in left ventricular diastolic dimension while the left ventricular systolic dimension was unchanged or due to a decreased preload with an increase in afterload after ductal closure (Campbell et al, 2006). However, without afterload correction, the contractility of left ventricle may actually not be depressed. A decrease of fractional shortening and ejection may persist for a long period of time after ductal occlusion without clinical significance (Stauthammer et al, 2013). Moreover, mitral regurgitation is a common finding in dogs with a PDA (Van Israël et al, 2003). Mitral regurgitation still could be seen in large number of dogs after ductal closure for a period of 9 to 121 months of study (Van Israël et al, 2003). This is probably secondary to the degenerative changes of valve leaflets due to high volume and velocity flow across the valve (Van Israël et al, 2003), myocardial dysfunction after ductal closure or mitral annular dilation and papillary muscle displacement from volume overload (Kittleson, 1998). Aortic insufficiency may remain unchanged after ductal closure (Saunders et al, 2007). This is probably because of the valve damage or the aortic dilatation. An immediate stop of the volume overload can be seen after successful complete ductal occlusion (Stauthammer et al, 2013) as Buranakarl C. et al. / Thai J Vet Med. 2015. 45(4): 593-602. seen in this dog of which the size of left atrium and ventricle was decreased after device occlusion for 3 days. Six months after device occlusion, the clinical signs of dog were improved to nearly normal. The echocardiographic data showed the reverse remodeling in both anatomical and mechanical functions. Although the data revealed lower FS than before PDA correction, the improved cardiac function with slow heart rate and reduced preload overcome the lower FS to ensure that the progression of heart failure was not compromised. The process of PDA occlusion by transcatheter method has become more common in specialist animal teaching hospital in universities worldwide. This procedure is preferred over traditional method (i.e. thoracotomy) because it has rapid post intervention recovery and less postoperative complications due to avoidance of chest surgery. However, this procedure requires appropriate facilities, instruments (i.e. fluoroscope, coil, or occluder), and experienced operators. The transcatheter method is also more expensive than the open-chest surgery but it is worth for the patients and owners. In conclusion, a successful interventional treatment was reported in dog with large PDA. The dog was still full with high energetic and more exercise tolerance with reduction of left ventricle size and less valvular regurgitation within 1 month. The dog had schedule to reevaluate at 3 months and 1 year after PDA occlusion. References Boon J 2011. Congenital shunt and AV valve dysplasia. In: Veterinary echocardiography. 2nd edition. Ames; Wiley-Blackwell. p 562-673. Buchanon JW 1992. Causes and prevalence of cardiovascular disease. In: Kirk’s Current Veterinary Therapy. 11th edition. Saunders. p 647655. Campbell FE, Thomas WP, Miller SJ, Berger D and Kittleson MD 2006. Immediate and late outcomes of transrterial coil occlusion of patent ductus arteriosus in dogs. J Vet Intern Med. 20:83-96. Fellows CG, Lerche P, King G and Tometzki A 1998. Treatment of patent ductus arteriosus by placement of two intravascular embolization coils in a puppy. J Small Ani Pract. 39:196-199. Fox PR, Bond RB and Summer RJ 1998. Non-surgical transcathter coil occlusion of patent ductus arteriosus in two dogs using a performed nitinol snare delivery technique. J Vet Intern Med. 12:182-185. GlausTM, Berger F, Ammann FW, Kiowski W, Ohlert S, Boller M, Kästner S, Reusch CE and Sisson D 2002. Closure of large patent ductus arteriosus with a self-expanding duct occlude in two dogs. J Small Ani Pract. 43:547-550. Gluaus TM, Gardelle O, Bass M and Kiowski WK 1999. Verschluss eines ductus arteriosus botalli bei zwei hnden mittels transarterieller coli embolisation. Schweizer Archiv für Tierheilkunde. 141:191-194. Buranakarl C. et al. / Thai J Vet Med. 2015. 45(4): 593-602. Grifka RG, Miller MW, Frischmeyer KJ and Mullins CE 1996. Transcatheter occlusions of a patent ductus arteriosus in a new foundland puppy using the Gianturco-Grifka vascular occlusion device. J Vet Intern Med. 10:42-44. Hamabe L, Kim S, Yoshiyuki R,Fukayama T, Nakata TM, Fukushima R and Tanka R 2015. Echocardiographic evaluation of myocardial changes observed after closure of patent ductus arteriosus in dogs. J Vet Intern Med. 29:126-131. Kittleson M. Patent ductusarteriosus 1998. In: Kittleson M, Kienle R, eds. Small animal Cardiovascularmedicine. St. Louis: Mosby. p 218–230. Miller MW, Stephien RL, Meurs KM and Boswood A 1994. Echocardiographic assessment of patent ductus arteriosus (PDA) after occlusion. Proceedings of the 14th Annual Veterinary Forum, ACVIM, San Francisco, CA. 305-306. Nguyenba TP and Tobias AH 2008. Minimally invasive per-catheter patent ductus arteriosus occlusion in dogs using a prototype duct occluder. J Vet Intern Med. 22:129–34. Saunders AB, Gordon SG, Boggess MM and Miller MW 2014. Long-term outcome in dogs with patent ductus arteriosus: 520 cases (1994-2009). J Vet Intern Med. 28:401-410. Saunders AB, Miller MW, Gordon SG and Bahr A 2007. Echocardiographic and angiographic comparison of ductal dimensions in dogs with patent ductus arteriosus. J Vet Intern Med. 21:68–75. Schneider M, Hagel KJ, Schneider I and Neu H 1996. Transvenöse coil-embolisaion zum verschluss des persistierenden ductus arteriosus (botalli) bei einem hund. Kleintierpraxis. 41:685-691. Schneider M, Hildebrandt N, Schweigl T and Wehner M 2007. Transthoracic echocardiographic measurement of patent ductus arteriosus in dogs. J Vet Intern Med. 21:251–257. Singh MK, Kittleson MD, Kass PH and Griffiths LG 2012. Occlusion devices and approaches in canine patent ductus arteriosus: comparison of outcomes. J Vet Intern Med. 26:85-92. Snaps FR, McEntee K, Saunders JH and Dondelinger RF 1995. Treatment of patent ductus arteriosus by placement of intravascular coils in a pup. J Am Vet Med Assoc. 207:724-725. Staunthammer CD, Tobias AH, Leeder DB and Kruger MU 2013. Structural and functional cardiovascular changes and their consequences following interventional patent ductus arteriosus occlusion in dogs: 24 cases (2000-2006). J Am Vet Med Assoc. 242:1722-1726. Stauthammer CD 2015. Chapter Fifty-Eight: Patent ductus arteriosus. In: Veterinary Image-Guided Interventions. 1st edition. John Wiley & Sons, Inc. p 564-574. Van Israël N, Dukes-McEwan J and French AT 2003. Long-term follow-up of dogs with patent ductus arteriosus. J Small Ani Pract. 44:480–490. Van Israël N, French AT, Dukes-McEwan J and Corcoran BM 2002. Review of left-to-right shunting patent ductus arteriosus and short term outcome in 98 dogs. J Small Ani Pract. 43:395–400. 601 600 602 Buranakarl C. et al. / Thai J Vet Med. 2015. 45(4): 593-602. บทคัดย่อ การอุดหลอดเลือดพีดีเอ (Patent Ductus Arteriosus) โดยใช้เทคนิกอินเตอร์เวนชันผ่านหลอด เลือดดาในสุนัข ชลลดา บูรณกาล1* อนุศักดิ์ กิจถาวรรัตน์1 วสันต์ อุทัยเฉลิม2 สิริลักษณ์ สุรเชษฐพงษ์3 ทรายแก้ว สัตยธรรม1 ภาสกร พฤกษะวัน4 สุวิชา จุฑาเทพ4 ราไพภัทร ตั้งจิตเพียรพงศ์5 นาฏทิวา ไชยวรวิทย์สกุล5 การแก้ไขพีดีเอ (Patent Ductus Arterious; PDA) โดยใช้วิธีอินเตอร์เวนชันครั้งแรกในประเทศไทยกระทาในสุนัขพันธุ์โกลเดน รีท รีฟเวอร์อายุ 2 ปี สุนัขเข้ารับการรักษาโดยตรวจพบเสียงผิดปกติของการเต้นของหัวใจแบบต่อเนื่อง มีภาวะหัวใจข้างซ้ายโตร่วมกับภาวะลิ้น หัวใจพัลโมนิก ไมตรัลและเอออร์ติกรั่ว การตรวจวินิจฉัยได้รับการยืนยันว่ามีพีดีเอ ด้วยการตรวจคลื่นเสียงสะท้อนความถี่สูงที่หัวใจ สุนัขได้รับ การแก้ไขพีดีเอด้วยวิธีอินเตอร์เวนชัน โดยใช้อุปกรณ์ที่ประกอบด้วยตาข่าย นิทิโนล (nitinol mesh) โดยกระทาผ่านกล้องฟรูโอโรสโคป ณ โรงพยาบาลสัตว์เล็ก คณะสัตวแพทยศาสตร์ จุฬาลงกรณ์มหาวิทยาลัย ด้วยความร่วมมือกับผู้เชี่ยวชาญ คณะแพทยศาสตร์ จุฬาลงกรณ์ มหาวิทยาลัย การรักษาครั้งนี้ประสบความสาเร็จเป็นอย่างดี โดยอุปกรณ์อุดได้สมบูรณ์และไม่พบอาการแทรกซ้อนหลัก สุนัขแข็งแรงเป็นปกติ และเมื่อตรวจด้วยคลื่นเสียงสะท้อนความถี่สูงพบว่า ขนาดหัวใจล่างซ้ายเล็กลงร่วมกับการรั่วของลิ้นหัวใจทั้งสามที่ลดลงภายหลังการแก้ไขครบ 1 เดือน คาสาคัญ: สุนัข ตัวอุดพีดีเอ เทคนิกอินเตอร์เวนชัน 1ภาควิชาสรีรวิทยา 3ภาควิชาอายุรศาสตร์ 4ภาควิชาศัยศาสตร์ 5โรงพยาบาลสัตว์เล็ก คณะสัตวแพทยาสตร์ จุฬาลงกรณ์มหาวิทยาลัย 2ภาควิชาสรีรวิทยา คณะแพทยศาสตร์ จุฬาลงกรณ์มหาวิทยาลัย ปทุมวัน กรุงเทพ ฯ 10330 *ผู้รับผิดชอบบทความ E-mail: [email protected]