* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Large Left Ventricle, Paradoxically Split Second Heart Sound, and a
Heart failure wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Electrocardiography wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Atrial septal defect wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
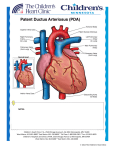
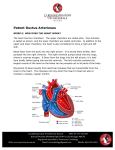
Journal of the Louisiana State Medical Society ECG OF THE MONTH Large Left Ventricle, Paradoxically Split Second Heart Sound, and a Continuous Murmur C. Carmen Ilie, MD; Roberto E. Quintal, MD; Neeraj Jain, MD; Murtuza J. Ali, MD; Christopher Daniels, MD; Frederick R. Helmcke, MD; and D. Luke Glancy, MD An asymptomatic, muscular, 41-year-old man came to the clinic because of a swishing sound in his ears at night. On exam his neck veins were normal; pulses were full and brisk; and a large and dynamic left ventricular impulse was felt just outside the left anterior axillary line in the sixth intercostal space. The second heart sound was paradoxically split, and both components were loud. A prominent third heart sound was heard at the cardiac apex, and a grade 4/6 continuous murmur was best heard in the second left intercostal space. An electrocardiogram was recorded (Figure 1). Figure 1. Electrocardiogram recorded at one-half standard, where 5 mm = 1mV, of a 41-year-old man with a continuous murmur. See text for explication. What is your diagnosis? Explication is on page 228. 226 J La State Med Soc VOL 163 July/August 2011 Journal of the Louisiana State Medical Society ECG of the Month Presentation is on page 226. DIAGNOSIS: Sinus bradycardia, left bundle branch block, and QRS voltage of left ventricular enlargement. The paradoxically split second heart sound predicts left bundle branch block (LBBB). The commonest cause of LBBB is left ventricular enlargement, which here is documented by the size and position of the left ventricular impulse and is emphasized by the QRS voltage. There are many causes of continuous murmurs.1 A good rule is that if it is maximal between the left second intercostal space and the left clavicle and peaks at the second heart sound, the continuous murmur, known as Gibson’s murmur,2 is due to a patent ductus arteriosus (PDA), whereas if the continuous murmur is maximal elsewhere it is probably due to some other cause – coronary arteriovenous fistula, ruptured sinus of Valsalva aneurysm, etc. Thus, the location of this patient’s murmur indicates a PDA. Undiagnosed patent ductus arteriosus is rare in adults of developed countries, but less so in immigrant laborers such as this man. The diagnosis was confirmed by echo-Doppler examination and computed tomographic angiography which showed a ductus 7 mm in diameter, ie, ~ 0.4 cm2 in cross-sectional area, with continuous flow through it into the left pulmonary artery just beyond its origin from the pulmonary trunk (Figure 2). A ductus this size results in a large left-to-right shunt with the extra workload being borne almost entirely by the left ventricle, which accounts for that chamber’s huge internal diameter (8.5 cm in diastole) in this patient. The left ventricle in such patients eventually fails,3 and the ejection fraction of only 40% suggests that this will occur soon rather than later in this patient. Pulmonary arterial systolic pressure is estimated to be 35-45 mm Hg (reference ≤ 30); there is mild pulmonic regurgitation; and the right ventricle has normal size and function. Patent ductus arteriosus is a mechanical problem that lends itself to a mechanical solution, ie, closure of the ductus. Robert Gross first accomplished this surgically over 70 years ago.4 Initially this was performed by simple ligation, but because of a significant recurrence rate with that procedure, suture-ligation and division of the ductus became the method of choice. More recently, catheter-based closure of the ductus, initially done with coils5 and subsequently with the Amplatzer duct occluder,6 has become the procedure of choice. The Amplatzer Duct Occluder, frequently used for closure of moderate to large PDAs, has two important features: a relatively small delivery system (5-7 Fr) and the ability to be retrieved and repositioned prior to detachment. A lateral angiogram with contrast injection in the descending aorta (Figure 2) is helpful in selecting the diameter of the device: at least 2 mm larger than the narrowest PDA diameter (5.9 mm in our case). Using a device 10 mm in diameter on the aortic side and 8 mm in diameter on Figure 2. Preclosure images of the patent ductus arteriosus (PDA). A. Color Doppler demonstrating flow from descending aorta (DAo) into pulmonary artery (PA) through the PDA. RV – right ventricle. B. Computed tomographic angiogram, axial view, showing the communication between the DAo and PA. AAo – ascending aorta. C. Aortogram, left lateral view, demonstrating contrast filling the PA through the PDA back to the pulmonary valve (black arrows). 228 J La State Med Soc VOL 163 July/August 2011 Figure 3. Postclosure images of the ductus arteriosus. A. Color Doppler showing the Amplatzer Duct Occluder (ADO) and the absence of residual shunt. RV – right ventricle, PA – pulmonary artery, DAo – descending aorta. B. Aortogram, left lateral view demonstrating the absence of residual shunt. the pulmonary arterial side, we achieved complete PDA occlusion with no residual shunt as judged by angiography and echocardiography (Figure 3). REFERENCES 1. 2. 3. 4. 5. 6. Marquis RM. Persistence of the ductus arteriosus. In: Watson H (editor). Paediatric Cardiology. St. Louis: C.V. Mosby;1968:242-285. Gibson GA. Diseases of the Heart and Aorta. Edinburgh: Young J. Pentland;1898:161. Campbell M. Patent ductus arteriosus. Some notes on prognosis and pulmonary hypertension. Brit Heart J 1955;17:511-533. Gross RE, Hubbard JP. Surgical ligation of a patent ductus arteriosus. Report of first successful case. JAMA 1939;112:729-731. Alwi M, Kang LM, Samion H, et al. Transcatheter occlusion of native patent ductus arteriosus using conventional Gianturco coils. Am J Cardiol 1997;79:1430-1432. Masura J, Walsh KP, Thanopoulous B, et al. Catheter closure of moderate-to-large-sized patent ductus arteriosus using the new Amplatzer duct occluder: immediate and short-term results. J Am Coll Cardiol 1998;31:878-882. Dr. Ilie is an interventional fellow in the Section of Cardiology, Department of Medicine, Louisiana State University Health Sciences Center, New Orleans (LSUHSC-NO). Dr. Quintal is a clinical professor of medicine in the Section of Cardiology, Department of Medicine, LSUHSC-NO. Drs. Jain and Ali are assistant professors of clinical medicine in the Section of Cardiology, Department of Medicine, LSUHSC-NO. Dr. Daniels is an interventional fellow in the Section of Cardiology, Department of Medicine, LSUHSC-NO. Dr. Helmcke is an assistant professor of clinical medicine in the Section of Cardiology, Department of Medicine, LSUHSC-NO. Dr. Glancy is a professor in the Section of Cardiology, Department of Medicine, LSUHSC-NO. J La State Med Soc VOL 163 July/August 2011 229