* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 722_Wk03_VitaminA
Survey
Document related concepts
Transcript
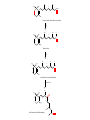
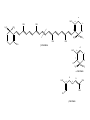
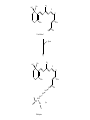
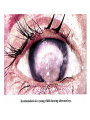
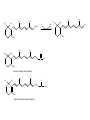
Phar 722 Pharmacy Practice III VitaminsVitamin A Family Spring 2006 Vitamin A Study Guide • • • • • • • • • • • • • The applicable study guide items in the Vitamin Introduction. History Structures of the vitamin’s active forms Structures of the vitamin and its commercial forms Transport of the vitamin The advantage of Vitamin A esters in dosage forms The effects of structural changes on the activity of the vitamin A group including retinoid and retinoid-like drugs used to treat acne and psoriasis Role in the visual process Role in cell differentiation Symptoms and occurrences of hypervitaminosis A and hypercarotenosis Approaches to formulation Non-vitamin drug uses (It is not necessary to know drug names.) Dietary forms of the vitamin Vitamin A History • 1913 – McCollum, Davis, Osborne, and Mendel noted growth failure in rats fed purified rations with lard or olive oil as the source of lipid. • Growth resumed when butterfat, cod liver oil, or egg yolk was substituted for the lard or olive oil. • The active ingredient was called Fat Soluble A to differentiate it from Water Soluble B. • The growth factor was shown to be absent from cereal grains but present in alfalfa and cabbage beans and in ether extracts of spinach leaf and clover. • 1919 – Steenbock at the University of Wisconsin pointed that the vitamin A potency of certain plant sources seemed to run parallel with the amount of yellow, fat-soluble pigments present in them. • He suggested that the vitamin A activity might be associated with the carotenoid pigments. • Since cod liver oil concentrates are colorless, but very potent in vitamin A activity, Steenbock postulated (correctly) that the vitamin A of animals might be a colorless form of carotene. • 1930 – It was shown that ingested carotene is converted to vitamin A in the rat. This established the relationship to the active yellow carotene of plants and the nearly colorless highly active vitamin concentrates from fish liver oils. Vitamin A Chemistry-1 • Retinol – The all trans retinol is colorless and is obtained from animal sources, but animals cannot biosynthesize it. – In general, animals, including humans, make the vitamin by cleavage of the plant pigments known as the carotenes. • All animals have to obtain their vitamin A by eating other animals or from the consumption of plants. – Most vitamin A, whether in food or vitamin supplements, is found in the ester form. • See discussion on dosage forms. CH3 H3C CH3 CH3 11 CH2OH 13 9 CH3 Retinol (inactive commercial form esterified) [O] CH3 H3C CH3 CH3 O 11 CH 13 9 CH3 Retinal (vision) [O] CH3 H3C CH3 CH3 O 11 COH 13 9 CH3 trans-Retinoic Acid (cell differentiation) isomerization CH3 H 3C CH3 9 11 CH3 13 H 3C 9-cis-Retinoic Acid (cell differentiation) COH O Vitamin A Chemistry-2 • Carotenes – These are the yellow/orange pigments which can be considered as provitamins A. – There are three main pigments with βcarotene considered the standard because each mole contains two equivalents of vitamin A. – The carotenes are oxidatively cleaved in the intestinal mucosa cell. d H3 C CH3 H3 C e c CH3 CH3 f b a H3 C CH3 CH3 CH3 CH3 -Carotene d H3 C e c f b R H3C a CH3 -Carotene d H3 C e b CH3 c a CH3 f R -Carotene Vitamin A Uptake and Metabolism • Vitamin A esters are hydrolyzed in the intestine, and the vitamin absorbed with other lipid material. • Reesterification takes place in the mucosal cell and the final product transported on the chylomicrons to the liver where it is stored. Subsequent distribution from the liver occurs as retinal bound to a special retinal binding protein (RBP). • Carotenes are absorbed in the mixed micelles into the mucosal cells where they are cleaved to the aldehyde, reduced to the alcohol and esterified. • At this point, the new vitamin A esters follow the same distribution scheme outlined for vitamin A, itself. • The absorption of the carotenes is poor relative to vitamin A. There is a positive correlation between the fat content of the diet and carotene absorption. oxidation carotene retinal mucosa cell mucosa cell reductase retinol reesterify retinol palmitate mucosa cell Retinol esters (food or vitamin supplements) intestinal esterases TGs retinal & retinoic acid transported on Retinal Binding Protein (RBP) (1) esterase (2) oxidation liver storage chylomicron remnent chylomicron Relationship between Protein Malnutrition and Vitamin A Status • Patients with kwashiorkor and other protein malnutrition states have very low serum vitamin A levels. – This is because there is a deficiency of the amino acids necessary to produce the retinal binding protein. – Administration of protein supplements will increase serum vitamin A levels PROVIDED there is sufficient amounts of the vitamin in the patient's liver. – There are reports of precipitating a vitamin A deficiency in protein malnourished patients who are given protein supplements. Today, it is common to use vitamin A fortified powdered milks to insure that there will be adequate levels of the vitamin in the patient's diet. – Today, our milk is fortified with both vitamins A and D. Vitamin A Deficiency-1 • Keep in mind that it is difficult to study the effects of vitamin A deficiencies in otherwise healthy humans because there is enough of the vitamin stored in the liver to last 6 - 9 months. Vitamin A Deficiency-2 Retinoic Acid - Cell differentiation The retinoic acid receptors that belong to the nuclear receptor family and are classified as – RARα,β,γ (ligand: trans-retinoic acid) – RXRα,β,γ (ligand: 9-cis-retinoic acid) • Retinoic acids are required for cell differentiation in the developing embryo. – A preliminary report (mice) indicates that retinoic acid appears to control the timing and perhaps the choice of germ cells to begin changing into eggs or sperm. • Retinoic acid now is considered a hormone that plays a pivotal role in cell differentiation in embryos. Improperly used, it is teratogenic. – One metabolic defect in utilizing retinoic acid apparently leads to acute promyelocytic leukemia. This is a rare leukemia with about 1,000 new patients in the United States each year. – Administration of the all-trans retinoic acid causes remissions in well over half of the patients. – One hypothesis is that the retinoic acid causes the immature leukemic cell to mature, at least to the level it stops dividing. Vitamin A Deficiency-3 Retinoic Acid - Cell differentiation • Deficiencies affect cells of the skin, cornea, lungs and digestive tract. – The vitamin, as retinoic acid, is required for the development of goblet or mucous secreting cells. – An absence of the vitamin leads to keratinization of this tissue. – There is some evidence that the vitamin affects certain aminoacyl tRNA synthetase enzymes needed for the glycoproteins found in mucous. Specific mucous glycoproteins are missing in vitamin A deficient individual. – For adults, this is the most serious form of the deficiency because the mucous layer forms one of the physical barriers to microbial infection. A vitamin A deficient individual can die from infection. • Bear in mind that this same individual probably is deficient in several nutrients and may have a compromised immune system. • The patient's skin can have a goose bump or acne like appearance. – This has led to the suggestion that vitamin A may be effective in the treatment of acne. With the exception of the specific local activity of the retinoic acids, there is no evidence that acne is related to vitamin A status in the patient. Vitamin A and Vision • Vitamin A is required for vision. – It is part of the visual pigments found in the rods. • The visual pigment in the rods is known as rhodopsin. The rods are required for night vision. – In bright light, such as you are using while reading this outline, the cones are functioning. – Try this exercise. • Go from a brightly lit room into a closet. You probably will experience what appear to be flashes of light. Then you will notice light coming in from around the cracks in the closet door. The time it takes for you to perceive light from around the cracks in the door is known as the accommodation time. • The rhodopsin has formed in the rods from opsin and 11-cis retinal and then cleaves back to opsin and all-trans retinal as light reaches the rods. These changes are transmitted to the brain by the optic nerve. – Notice that you cannot perceive color in very dim light. – Now step back into the lighted room. It may seem painful and you will try to shield your eyes. • There has been a rapid, massive conversion of rhodopsin back to opsin and the all-trans retinal. The rhodopsin will not form again until subdued light return. Rhodopsin (visual pigment) (Light) Changes in the conformation of the Rhodopsin complex and hydrolysis of the enamine. (Dark) 11-cis-Retinal + Opsin Nerve impulse Sight to the brain trans-Retinal + Opsin 11-cis-Retinol trans-Retinol (transported to the eye from the liver on a retinol binding protein) Liver stores of retinol esters H3C CH3 CH3 11 12 CH3 CH3 O H 11-cis-Retinal Opsin CH3 CH3 H3C 11 12 CH3 CH3 N H2C H2C H2C HC C O NH Rhodopsin Lys H Vitamin A Deficiency and Vision • Night blindness results from delayed accommodation time. A deficiency of vitamin A means that there will be a lack of retinal to combine with the opsin. • Xerophthalmia is the most tragic aspect of vitamin A deficiency. This is the result of the vitamin deficiency in children. There is irreversible deterioration of the developing eye which causes blindness. There are 3 - 10 million children with this condition. Within this group 250,000 - 500,000 still go blind annually go blind because of a vitamin deficiency that could be prevented by just a few cents a year. Hypervitaminosis A – Acute-1 • This is rare and is somewhat dependent on the dosage form. The rate of absorption is: – Greatest for aqueous preparations • Vitamin A, being oil soluble, must be dispersed by Tweens to produce an aqueous preparation. Since it already is emulsified, it will be rapidly and efficiently absorbed into the intestinal mucosa. – Intermediate for standard emulsions – Slowest for oil solutions including IM injections. • Therefore, a toxic dose for an aqueous preparation may be a safe dose for an oil solution. Hypervitaminosis A – Acute-2 Examples – A child swallowing 50 ml of fish oil concentrate containing 3,000,000 IU (900,000 μg or 900 mg) of vitamin A experienced nausea and vomiting. (1 IU of vitamin A activity equals 0.3 μg of alltrans-retinol) – 300,000 IU (90 mg) would probably cause acute hypervitaminosis A in infants – An IM injection of 1,000,000 IU of water miscible vitamin A might show a transient hypervitaminosis A depending on the child's age and nutritional state. – Polar bear liver • 18,000-35,000 IU/gm • A diet by a lost arctic explorer would consist of 100-500 gm of liver containing 1,800,000-17,500,000 IU (540 - 5,250 mg). • The symptoms for acute hypervitaminosis A include headache, vertigo, diarrhea, nausea, and vomiting. Recovery requires about 2-4 weeks. Hypervitaminosis A – Chronic-1 • This is the more common form of hypervitaminosis A and can be the result of a parent administering too much of the vitamin to a child or teenagers with acne taking very large doses of the vitamin. • Examples – 23 month male receiving 250,000 IU/day (75 mg/day) for 20 months – 14 month male receiving 125,000 IU/day (37.5 mg/day) for 13 months – 9 month female receiving 220,000 IU/day (66 mg/day) for 8 months • Most of these infants received fish liver oil concentrates that contained both vitamins A and D. • Adults receiving 100,000 - 600,000 IU/day (30 - 180 mg) for months and years. – Symptoms will not appear until the binding capacity of the liver is exhausted. Hypervitaminosis A – Chronic-2 • Symptoms – fatigue, malaise, lethargy, abdominal discomfort, bone/joint pain, severe and throbbing headache, insomnia, restlessness, dry and scaly skin, loss of body hair, brittle nails, constipation, irregular menses. – This is a nondescript group of symptoms that could cause the patient to increase the dose even further. • A characteristic of patients with any hypervitaminosis is to not disclose to the physician that they are taking large amounts of a vitamin(s). • There are reports that cirrhosis of the liver can develop if the intake of excessive vitamin A is not reduced to normal levels. • Nontraumatic Hip Fracture – The Nurses’ Health Study has reported that women with the highest intake of vitamin A (as retinol) had the higher rates of nontraumatic hip fracture.1 There is evidence that long-term intake of retinol stimulates bone resorption and inhibits bone formation, therefore, contributing to osteoporosis and hip fractures. • Teratogenesis in pregnancies – There are warnings about taking high doses of vitamin A during pregnancy. Hypercarotenosis • This occurs from massive doses of carotene which exceed the capacity of the mucosa cells to cleave the molecule to retinal derivatives. – The excess carotene becomes deposited in the body tissues. – Except for the yellow skin, there seem to be no other symptoms. The skin coloration will slowly disappear when carotene intake stops. • Solatene™ capsules contain 30 mg β-carotene and are indicated for the photosensitivity seen in erythropoietic porphyria. • Patients who drink large amounts of carrot juice sometimes show signs of hypercarotenosis. • β-Carotene was included in several vitamin products and was promoted for its antioxidant properties. – Most studies on the use of EXCESSIVE β-carotene show it to be ineffective and may be detrimental. Dosage Formulations-1 • Commercial forms – Retinol – Retinol Acetate – Retinol Palmitate • Stability – Vitamin A is one of the more unstable vitamins. It is sensitive to • acid - rapidly dehydrates • oxygen - due to the high degree of unsaturation • UV light - due to the high degree of unsaturation – Therefore, the vitamin must be protected from light by protective coatings, from oxygen with antioxidants, and acid dehydration by esterification. CH3 H3C CH3 CH3 CH3 H2O H+ H3C CH2+ CH2OH CH3 CH3 H3C CH3 CH3 O O C CH3 CH3 Vitamin A Acetate; Retinol Acetate CH3 H3C CH3 CH3 O O CH3 Vitamin A Palmitate; Retinol Palmitate C C15H31 CH3 CH3 CH3 + Dosage Formulations-2 • Oral dosage forms – Sealed gelatin capsules – Oil solutions – Water dispersible (Tweens) liquids sometimes knows as clear emulsions. – Granulations used in variety of dry dosage forms. • The vitamin is dissolved in a volatile solvent and sprayed onto a gelatin-sugar matrix. After the solvent is removed, the coated gelatin material is powdered. The result is a free flowing powder of an oily vitamin. • Intramuscular – Sterile aqueous dispersion of a vitamin A ester – Oil solutions DRIs (1 μg = 0.001 mg) • AI (infants 1 - 12 months) • EAR – – – – – – • 210 - 275 μg/day 445 - 630 μg/day 420 - 485 μg/day 625 μg/day 500 μg/day 880 - 900 μg/day RDA – – – – • Children (1 - 8 years) Boys (9 - 18 years) Girls (9 - 18 years) Men (19 - 70+ years) Women (19 - 70+) Lactating 400 - 500 μg/day Men Women Pregnant Lactating 900 μg/day 700 μg/day 770 μg/day 1,200 - 1,300 μg/day UL – 3,000 μg/day for all adults including pregnant women. There is some concern of teratogenic effects based on the experience of the retinoids used in therapy. There are warnings for women who plan on becoming pregnant or who are pregnant to not exceed the RDA for pregnancy. – Long-term intake of a diet high in retinol may promote the development of osteoporotic hip fractures. • JAMA, 287(10, 47-54, January 2, 2002 Food Sources • • • • Fish and animal liver Carotene containing vegetables Fortified milk Genetically modified rice (developed but not yet used; It is yellow because of the presence of β-carotene). Retinoid and Retinoid-like Drugs Indicated for Acne-1 CH3 H3C CH3 O CH3 C 13 CH3 OH Tretinoin (Retin-A) Retinoic Acid Topical: Produces a complex response related to increasing the turnover of follicular epithelial cells and decreasing the cohesiveness of follicular epithelial cells. Topical treatment does not affect plasma concentrations of retinol, retinyl esters or retinoic acids in female subjects of child-bearing age. Toxicology Letters, 163, 65-76, 2006. Retinoid and Retinoid-like Drugs Indicated for Acne-2 CH3 H3C CH3 CH3 13 C CH3 Oral: Isotretinoin (Accutane 13-cis-Retinoic Acid TM O ) OH Mechanism poorly understood. It can produce severe birth defects in the fetus of pregnant women taking the drug. Retinoid and Retinoid-like Drugs Indicated for Acne-3 O C OH H3CO H2 C C CH2 Adapalene (Differin GelTM) H2 C Topical: Binds to the retinoic acid nuclear receptor that modulates cell differentiation, keratinization and inflammatory responses. T o Retinoid and Retinoid-like Drugs Indicated for Acne and Psoriasis O C H3C C CH3 C Tazarotene Gel (TazoracTM) S See next slide for warnings! OCH2CH3 Tazarotene Gel-Warnings • Topical: Indicated for both acne and psoriasis. It is a prodrug converted to the active form by hydrolysis of the ester. – Possible Mechanism: It binds to all three RAR receptors and also inhibits epidermal ornithine decarboxylase. The latter is required for cell proliferation. • Risk: – While it is used topically and there appears to be minimal absorption if used over limited skin area, there is some absorption with retention by the body for up to three months. It can cause fetal damage and cannot be used by pregnant women or women who may become pregnant. – It increases the skin's sensitivity to sun, and a sunscreen of at least SPF 15 should be used if the patient will be outside in direct sunlight. • New Indication: – Tazarotene, marketed as Avage™, has been approved to treat wrinkles and photodamaged skin. Retinoids Used in the Treatment of Psoriasis CH3 CH3 H3C CH3 O C OR H3CO CH3 Etretinate (TegisonTM); R = CH2CH3 Acitretin (SoriateneTM); R = H See next slide for information and warnings. Etretinate and Acitretin • Both drugs are orally administered and require that female patient have: – received oral and written warnings taking these drugs during pregnancy; – received oral and written warnings about risk of contraception failure; – been advised of the need to use two reliable forms of contraception simultaneously both during therapy and for at least three years following discontinuation of therapy. • The exceptions are for women who have had a hysterectomy or practices abstinence. The woman must acknowledge her understanding in writing. • Etretinate: This is a prodrug that is metabolized to acitretin. – "Terminal" half-life after six months of therapy: 120 days – There are boxed warnings regarding women who are pregnant or may become pregnant. Pregnancy tests are required before administration. • • Acitretin: Because of its shorter half-life, it is recommended for women of child-bearing age. – "Terminal" half-life: 33 – 96 hours Retinoids Used in the Treatment of Malignancies CH3 H3 C CH3 Topical: This retinoid binds RXR nuclear receptor families. 9 CH3 Alitretinoin (PanretinTM) 9-cis-Retinoic Acid H3C C O OH It is used in the treatment of Kaposi’s sarcoma, a malignant tumor usually involving the skin and commonly encountered in HIVpositive patients. An oral dosage form is under evaluation for psoriasis and a variety of cancers. The trade name, Panretin, refers to its ability to bind to all six known intracellular retinoid/retinoic acid subtypes. Retinoids Used in the Treatment of Malignancies CH2 H3C CH3 C OH CH3 H3C C CH3 O Bexarotene (TargretinTM) This “rexinoid” binds to the RXR, RAR and VDR nuclear receptor families. Indication: Refractory cutaneous T cell lymphoma. Oral with warnings regarding teratogenesis. “Retinoid” Drugs in Trial OH CH3 H3C CH3 H C CH3 H C C C H CH3 HN C C H C C H O Fenretinide; Retinamide This retinoid is in trials for recurring breast cancer, neuroblastoma, ovarian cancer and other malignancies. Like the other retinoid-based drugs, it combines with the RXR and RAR families and, presumably other nuclear receptors. It would cause developing cells to mature rather than revert to “immature” or “juvenile-like” malignant cells.